High mobility group Box 1 inhibits human pulmonary artery endothelial cell migration via a Toll-like receptor 4- and interferon response factor 3-dependent mechanism(s)
- PMID: 23148224
- PMCID: PMC3543019
- DOI: 10.1074/jbc.M112.434142
High mobility group Box 1 inhibits human pulmonary artery endothelial cell migration via a Toll-like receptor 4- and interferon response factor 3-dependent mechanism(s)
Abstract
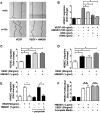
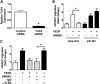
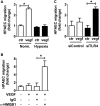
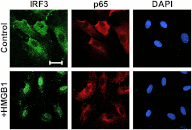
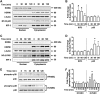
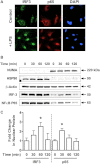
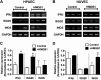
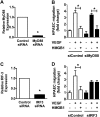
In pulmonary hypertension the loss of precapillary arterioles results from vascular injury causing endothelial dysfunction. Endothelial cell migration and proliferation are critical for vascular regeneration. This study focused on the effect of high mobility group box 1 protein (HMGB1) on these critical processes. HMGB1 had no effect on human pulmonary artery endothelial cell (HPAEC) proliferation. In contrast, treatment of HPAECs with HMGB1 dose-dependently inhibited VEGF-stimulated HPAEC migration. The effect of HMGB1 on HPAEC migration was TLR4-dependent because it was reversed by TLR4 siRNA or TLR4-neutralizing antibody. Exposure of HPAECs to hypoxia caused translocation and release of HMGB1 and inhibition of HPAEC migration. The effect of hypoxia on HPAEC migration was mediated by HMGB1 because HMGB1-neutralizing antibody but not control IgG restored HPAEC migration. Likewise, TLR4 siRNA but not control siRNA reversed the inhibitory effect of hypoxia in HPAECs. The canonical TLR4 signaling pathway requires the adaptor protein MyD88 and leads to downstream NFκB activation. Interestingly, HMGB1 failed to stimulate NFκB translocation to the nucleus, but instead activated an alternative pathway characterized by activation of interferon response factor 3 (IRF3). This was in contrast to human umbilical vein endothelial cells in which HMGB1 stimulated nuclear translocation of NFκB but not IRF3. IRF3 siRNA, but not MyD88 siRNA, reversed the inhibitory effect of HMGB1 on HPAEC migration. These data demonstrate that HMGB1 inhibits HPAEC migration, a critical process for vascular regeneration, via TLR4- and IRF3-dependent mechanisms.
Figures

References
-
- Evankovich J., Cho S. W., Zhang R., Cardinal J., Dhupar R., Zhang L., Klune J. R., Zlotnicki J., Billiar T., Tsung A. (2010) High mobility group box 1 release from hepatocytes during ischemia and reperfusion injury is mediated by decreased histone deacetylase activity. J. Biol. Chem. 285, 39888–39897 - PMC - PubMed
-
- Park J. S., Svetkauskaite D., He Q., Kim J. Y., Strassheim D., Ishizaka A., Abraham E. (2004) Involvement of Toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J. Biol. Chem. 279, 7370–7377 - PubMed
-
- Park J. S., Gamboni-Robertson F., He Q., Svetkauskaite D., Kim J. Y., Strassheim D., Sohn J. W., Yamada S., Maruyama I., Banerjee A., Ishizaka A., Abraham E. (2006) High mobility group box 1 protein interacts with multiple Toll-like receptors. Am. J. Physiol. Cell Physiol. 290, C917–924 - PubMed
-
- Huang W., Tang Y., Li L. (2010) HMGB1, a potent proinflammatory cytokine in sepsis. Cytokine 51, 119–126 - PubMed
-
- Ohmori H., Luo Y., Kuniyasu H. (2011) Non-histone nuclear factor HMGB1 as a therapeutic target in colorectal cancer. Expert Opin. Ther. Targets 15, 183–193 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

