A novel role of sphingosine 1-phosphate receptor S1pr1 in mouse thrombopoiesis
- PMID: 23148237
- PMCID: PMC3501353
- DOI: 10.1084/jem.20121090
A novel role of sphingosine 1-phosphate receptor S1pr1 in mouse thrombopoiesis
Abstract
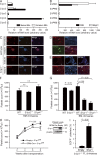
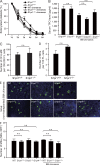
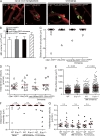
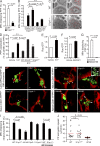
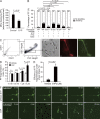
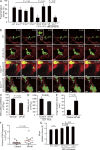
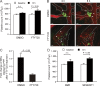
Millions of platelets are produced each hour by bone marrow (BM) megakaryocytes (MKs). MKs extend transendothelial proplatelet (PP) extensions into BM sinusoids and shed new platelets into the blood. The mechanisms that control platelet generation remain incompletely understood. Using conditional mutants and intravital multiphoton microscopy, we show here that the lipid mediator sphingosine 1-phosphate (S1P) serves as a critical directional cue guiding the elongation of megakaryocytic PP extensions from the interstitium into BM sinusoids and triggering the subsequent shedding of PPs into the blood. Correspondingly, mice lacking the S1P receptor S1pr1 develop severe thrombocytopenia caused by both formation of aberrant extravascular PPs and defective intravascular PP shedding. In contrast, activation of S1pr1 signaling leads to the prompt release of new platelets into the circulating blood. Collectively, our findings uncover a novel function of the S1P-S1pr1 axis as master regulator of efficient thrombopoiesis and might raise new therapeutic options for patients with thrombocytopenia.
Figures
References
-
- Avecilla S.T., Hattori K., Heissig B., Tejada R., Liao F., Shido K., Jin D.K., Dias S., Zhang F., Hartman T.E., et al. 2004. Chemokine-mediated interaction of hematopoietic progenitors with the bone marrow vascular niche is required for thrombopoiesis. Nat. Med. 10:64–71 10.1038/nm973 - DOI - PubMed
-
- Behnke O., Forer A. 1998. From megakaryocytes to platelets: platelet morphogenesis takes place in the bloodstream. Eur. J. Haematol. Suppl. 61:3–23 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

