Lipid functions in cytochrome bc complexes: an odd evolutionary transition in a membrane protein structure
- PMID: 23148267
- PMCID: PMC3497066
- DOI: 10.1098/rstb.2012.0058
Lipid functions in cytochrome bc complexes: an odd evolutionary transition in a membrane protein structure
Abstract
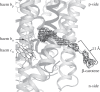
Lipid-binding sites and properties were compared in the hetero-oligomeric cytochrome (cyt) b(6)f and the yeast bc(1) complexes that function, respectively, in photosynthetic and respiratory electron transport. Seven lipid-binding sites in the monomeric unit of the dimeric cyanobacterial b(6)f complex overlap four sites in the Chlamydomonas reinhardtii algal b(6)f complex and four in the yeast bc(1) complex. The proposed lipid functions include: (i) interfacial-interhelix mediation between (a) the two 8-subunit monomers of the dimeric complex, (b) between the core domain (cyt b, subunit IV) and the six trans membrane helices of the peripheral domain (cyt f, iron-sulphur protein (ISP), and four small subunits in the boundary 'picket fence'); (ii) stabilization of the ISP domain-swapped trans-membrane helix; (iii) neutralization of basic residues in the single helix of cyt f and of the ISP; (iv) a 'latch' to photosystem I provided by the β-carotene chain protruding through the 'picket fence'; (v) presence of a lipid and chlorophyll a chlorin ring in b(6)f in place of the eighth helix in the bc(1) cyt b polypeptide. The question is posed of the function of the lipid substitution in relation to the evolutionary change between the eight and seven helix structures of the cyt b polypeptide. On the basis of the known n-side activation of light harvesting complex II (LHCII) kinase by the p-side level of plastoquinol, one possibility is that the change was directed by the selective advantage of p- to n-side trans membrane signalling functions in b(6)f, with the lipid either mediating this function or substituting for the trans membrane helix of a signalling protein lost in crystallization.
Figures


References
-
- Kurisu G., Zhang H., Smith J. L., Cramer W. A. 2003. Structure of the cytochrome b6f complex of oxygenic photosynthesis: tuning the cavity. Science 302, 1009–101410.1126/science.1090165 (doi:10.1126/science.1090165) - DOI - DOI - PubMed
-
- Yan J., Kurisu G., Cramer W. A. 2006. Structure of the cytochrome b6f complex: binding site and intraprotein transfer of the quinone analogue inhibitor 2,5-dibromo-3-methyl-6-isopropyl-p-benzoquinone. Proc. Natl Acad. Sci. USA 103, 67–7410.1073/pnas.0504909102 (doi:10.1073/pnas.0504909102) - DOI - DOI - PMC - PubMed
-
- Yan J., Kurisu G., Cramer W. A. 2006. Intraprotein transfer of the quinone analogue inhibitor 2,5-dibromo-3-methyl-6-isopropyl-p-benzoquinone in the cytochrome b6f complex. Proc. Natl Acad. Sci. USA 103, 69–7410.1073/pnas.0504909102 (doi:10.1073/pnas.0504909102) - DOI - DOI - PMC - PubMed
-
- Yamashita E., Zhang H., Cramer W. A. 2007. Structure of the cytochrome b6f complex: quinone analogue inhibitors as ligands of heme cn. J. Mol. Biol. 370, 39–5210.1016/j.jmb.2007.04.011 (doi:10.1016/j.jmb.2007.04.011) - DOI - DOI - PMC - PubMed
-
- Baniulis D., Yamashita E., Whitelegge J. P., Zatsman A. I., Hendrich M. P., Hasan S. S., Ryan C. M., Cramer W. A. 2009. Structure–function, stability, and chemical modification of the cyanobacterial cytochrome b6f complex from Nostoc sp. PCC 7120. J. Biol. Chem. 284, 9861–986910.1074/jbc.M809196200 (doi:10.1074/jbc.M809196200) - DOI - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

