Protein kinases and phosphatases involved in the acclimation of the photosynthetic apparatus to a changing light environment
- PMID: 23148273
- PMCID: PMC3497069
- DOI: 10.1098/rstb.2012.0064
Protein kinases and phosphatases involved in the acclimation of the photosynthetic apparatus to a changing light environment
Abstract
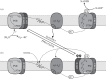
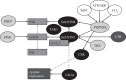
Photosynthetic organisms are subjected to frequent changes in light quality and quantity and need to respond accordingly. These acclimatory processes are mediated to a large extent through thylakoid protein phosphorylation. Recently, two major thylakoid protein kinases have been identified and characterized. The Stt7/STN7 kinase is mainly involved in the phosphorylation of the LHCII antenna proteins and is required for state transitions. It is firmly associated with the cytochrome b(6)f complex, and its activity is regulated by the redox state of the plastoquinone pool. The other kinase, Stl1/STN8, is responsible for the phosphorylation of the PSII core proteins. Using a reverse genetics approach, we have recently identified the chloroplast PPH1/TAP38 and PBPC protein phosphatases, which counteract the activity of STN7 and STN8 kinases, respectively. They belong to the PP2C-type phosphatase family and are conserved in land plants and algae. The picture that emerges from these studies is that of a complex regulatory network of chloroplast protein kinases and phosphatases that is involved in light acclimation, in maintenance of the plastoquinone redox poise under fluctuating light and in the adjustment to metabolic needs.
Figures


References
-
- Andersson B., Andersson J. 1980. Lateral heterogeneity in the distribution of chlorophyll–protein complexes of the thylakoid membranes of spinach chloroplasts. Biochem. Biophys. Acta 593, 427–44010.1016/0005-2728(80)90078-X (doi:10.1016/0005-2728(80)90078-X) - DOI - DOI - PubMed
-
- Dekker J. P., Boekema E. J. 2005. Supramolecular organization of thylakoid membrane proteins in green plants. Biochim. Biophys. Acta 1706, 12–3910.1016/j.bbabio.2004.09.009 (doi:10.1016/j.bbabio.2004.09.009) - DOI - DOI - PubMed
-
- Niyogi K. K. 1999. Photoprotection revisited: genetic and molecular approaches. Annu. Rev. Plant. Physiol. Plant. Mol. Biol. 50, 333–35910.1146/annurev.arplant.50.1.333 (doi:10.1146/annurev.arplant.50.1.333) - DOI - DOI - PubMed
-
- Kapri-Pardes E., Naveh L., Adam Z. 2007. The thylakoid lumen protease Deg1 is involved in the repair of photosystem II from photoinhibition in Arabidopsis. Plant Cell 19, 1039–104710.1105/tpc.106.046573 (doi:10.1105/tpc.106.046573) - DOI - DOI - PMC - PubMed
-
- Komenda J., Barker M., Kuvikova S., de Vries R., Mullineaux C. W., Tichy M., Nixon P. J. 2006. The FtsH protease slr0228 is important for quality control of photosystem II in the thylakoid membrane of Synechocystis sp. PCC 6803. J. Biol. Chem. 281, 1145–115110.1074/jbc.M503852200 (doi:10.1074/jbc.M503852200) - DOI - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
