Evolutionarily conserved differences in pallial and thalamic short-term synaptic plasticity in striatum
- PMID: 23148315
- PMCID: PMC3591703
- DOI: 10.1113/jphysiol.2012.236869
Evolutionarily conserved differences in pallial and thalamic short-term synaptic plasticity in striatum
Abstract
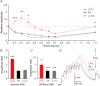
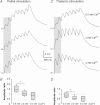
The striatum of the basal ganglia is conserved throughout the vertebrate phylum. Tracing studies in lamprey have shown that its afferent inputs are organized in a manner similar to that of mammals. The main inputs arise from the thalamus (Th) and lateral pallium (LPal; the homologue of cortex) that represents the two principal excitatory glutamatergic inputs in mammals. The aim here was to characterize the pharmacology and synaptic dynamics of afferent fibres from the LPal and Th onto identified striatal neurons to understand the processing taking place in the lamprey striatum. We used whole-cell current-clamp recordings in acute slices of striatum with preserved fibres from the Th and LPal, as well as tract tracing and immunohistochemistry. We show that the Th and LPal produce monosynaptic excitatory glutamatergic input through NMDA and AMPA receptors. The synaptic input from the LPal displayed short-term facilitation, unlike the Th input that instead displayed strong short-term synaptic depression. There was also an activity-dependent recruitment of intrastriatal oligosynaptic inhibition from both inputs. These results indicate that the two principal inputs undergo different activity-dependent short-term synaptic plasticity in the lamprey striatum. The difference observed between Th and LPal (cortical) input is also observed in mammals, suggesting a conserved trait throughout vertebrate evolution.
Figures


References
-
- Brodin L, Hokfelt T, Grillner S, Panula P. Distribution of histaminergic neurons in the brain of the lamprey Lampetra fluviatilis as revealed by histamine-immunohistochemistry. J Comp Neurol. 1990a;292:435–442. - PubMed
-
- Brodin L, Theordorsson E, Christenson J, Cullheim S, Hökfelt T, Brown J, Buchan A, Panula P, Verhofstad A, Goldstein M. Neurotensin-like peptides in the CNS of lampreys: chromatographic characterisation and immunohistochemical localisation with reference to aminergic markers. Eur J Neurosci. 1990b;2:1095–1109. - PubMed
-
- Buchwald NA, Price DD, Vernon L, Hull CD. Caudate intracellular response to thalamic and cortical inputs. Exp Neurol. 1973;38:311–323. - PubMed
-
- Calabresi P, Pisani A, Mercuri NB, Bernardi G. Long-term potentiation in the striatum is unmasked by removing the voltage-dependent magnesium block of NMDA receptor channels. Eur J Neurosci. 1992;4:929–935. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

