Functional expression of SK channels in murine detrusor PDGFR+ cells
- PMID: 23148317
- PMCID: PMC3577524
- DOI: 10.1113/jphysiol.2012.241505
Functional expression of SK channels in murine detrusor PDGFR+ cells
Abstract
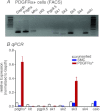
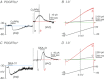
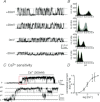
We sought to characterize molecular expression and ionic conductances in a novel population of interstitial cells (PDGFRα(+) cells) in murine bladder to determine how these cells might participate in regulation of detrusor excitability. PDGFRα(+) cells and smooth muscle cells (SMCs) were isolated from detrusor muscles of PDGFRα(+)/eGFP and smMHC/Cre/eGFP mice and sorted by FACS. PDGFRα(+) cells were highly enriched in Pdgfra (12 fold vs. unsorted cell) and minimally positive for Mhc (SMC marker), Kit (ICC marker) and Pgp9.5 (neuronal marker). SK3 was dominantly expressed in PDGFRα(+) cells in comparison to SMCs. αSlo (BK marker) was more highly expressed in SMCs. SK3 protein was observed in PDGFRα(+) cells by immunohistochemistry but could not be resolved in SMCs. Depolarization evoked voltage-dependent Ca(2+) currents in SMCs, but inward current conductances were not activated in PDGFRα(+) cells under the same conditions. PDGFRα(+) cells displayed spontaneous transient outward currents (STOCs) at potentials positive to -60 mV that were inhibited by apamin. SK channel modulators, CyPPA and SKA-31, induced significant hyperpolarization of PDGFRα(+) cells and activated SK currents under voltage clamp. Similar responses were not resolved in SMCs at physiological potentials. Single channel measurements confirmed the presence of functional SK3 channels (i.e. single channel conductance of 10 pS and sensitivity to intracellular Ca(2+)) in PDGFRα(+) cells. The apamin-sensitive stabilizing factor regulating detrusor excitability is likely to be due to the expression of SK3 channels in PDGFRα(+) cells because SK agonists failed to elicit resolvable currents and hyperpolarization in SMCs at physiological potentials.
Figures




Similar articles
-
UTP activates small-conductance Ca2+-activated K+ channels in murine detrusor PDGFRα+ cells.Am J Physiol Renal Physiol. 2015 Sep 15;309(6):F569-74. doi: 10.1152/ajprenal.00156.2015. Epub 2015 Jul 22. Am J Physiol Renal Physiol. 2015. PMID: 26202222 Free PMC article.
-
Purinergic inhibitory regulation of murine detrusor muscles mediated by PDGFRα+ interstitial cells.J Physiol. 2014 Mar 15;592(6):1283-93. doi: 10.1113/jphysiol.2013.267989. Epub 2014 Jan 6. J Physiol. 2014. PMID: 24396055 Free PMC article.
-
Platelet-derived growth factor receptor-α-positive cells and not smooth muscle cells mediate purinergic hyperpolarization in murine colonic muscles.Am J Physiol Cell Physiol. 2014 Sep 15;307(6):C561-70. doi: 10.1152/ajpcell.00080.2014. Epub 2014 Jul 23. Am J Physiol Cell Physiol. 2014. PMID: 25055825 Free PMC article.
-
Ca2+ dynamics in interstitial cells: foundational mechanisms for the motor patterns in the gastrointestinal tract.Physiol Rev. 2024 Jan 1;104(1):329-398. doi: 10.1152/physrev.00036.2022. Epub 2023 Aug 10. Physiol Rev. 2024. PMID: 37561138 Free PMC article. Review.
-
Small-conductance calcium-activated potassium channels in the heart: expression, regulation and pathological implications.Philos Trans R Soc Lond B Biol Sci. 2023 Jun 19;378(1879):20220171. doi: 10.1098/rstb.2022.0171. Epub 2023 May 1. Philos Trans R Soc Lond B Biol Sci. 2023. PMID: 37122223 Free PMC article. Review.
Cited by
-
Temporal sequence of activation of cells involved in purinergic neurotransmission in the colon.J Physiol. 2015 Apr 15;593(8):1945-63. doi: 10.1113/jphysiol.2014.287599. Epub 2015 Feb 23. J Physiol. 2015. PMID: 25627983 Free PMC article.
-
Cardiac PDGFRα+ interstitial cells generate spontaneous inward currents that contribute to excitability in the heart.FASEB J. 2023 May;37(5):e22929. doi: 10.1096/fj.202201712R. FASEB J. 2023. PMID: 37086093 Free PMC article.
-
UTP activates small-conductance Ca2+-activated K+ channels in murine detrusor PDGFRα+ cells.Am J Physiol Renal Physiol. 2015 Sep 15;309(6):F569-74. doi: 10.1152/ajprenal.00156.2015. Epub 2015 Jul 22. Am J Physiol Renal Physiol. 2015. PMID: 26202222 Free PMC article.
-
Regulation of PDGFRα+ cells and ICC in progesterone-mediated slow colon transit in pregnant mice.Heliyon. 2024 Jan 28;10(3):e25227. doi: 10.1016/j.heliyon.2024.e25227. eCollection 2024 Feb 15. Heliyon. 2024. PMID: 38333873 Free PMC article.
-
Downregulation of ICCs and PDGFRα+ cells on colonic dysmotility in hirschsprung disease.Front Pediatr. 2023 Jan 9;10:975799. doi: 10.3389/fped.2022.975799. eCollection 2022. Front Pediatr. 2023. PMID: 36699302 Free PMC article.
References
-
- Heppner TJ, Bonev AD, Nelson MT. Ca2+-activated K+ channels regulate action potential repolarization in urinary bladder smooth muscle. Am J Physiol. 1997;273:C110–C117. - PubMed
-
- Herrera GM, Etherton B, Nausch B, Nelson MT. Negative feedback regulation of nerve-mediated contractions by KCa channels in mouse urinary bladder smooth muscle. Am J Physiol Regul Integr Comp Physiol. 2005;289:R402–R409. - PubMed
-
- Herrera GM, Heppner TJ, Nelson MT. Voltage dependence of the coupling of Ca2+ sparks to BK(Ca) channels in urinary bladder smooth muscle. Am J Physiol Cell Physiol. 2001;280:C481–C490. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

