Chronic exposure of neonatal rat adrenomedullary chromaffin cells to opioids in vitro blunts both hypoxia and hypercapnia chemosensitivity
- PMID: 23148319
- PMCID: PMC3577520
- DOI: 10.1113/jphysiol.2012.243477
Chronic exposure of neonatal rat adrenomedullary chromaffin cells to opioids in vitro blunts both hypoxia and hypercapnia chemosensitivity
Abstract
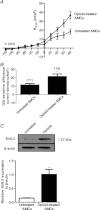
At birth, rat adrenomedullary chromaffin cells (AMCs) respond directly to asphyxial stressors such as hypoxia and hypercapnia by triggering catecholamine secretion, which is critical for proper transition to extrauterine life. These non-neurogenic responses are suppressed postnatally in parallel with the development of splanchnic innervation, and reappear following denervation of the adult adrenal gland. To test whether neural factors released from the splanchnic nerve may regulate AMC chemosensitivity, we previously showed that nicotinic agonists in utero and in vitro suppressed hypoxia, but not hypercapnia, sensitivity. Here, we considered the potential role of opiate peptides which are also released from the splanchnic nerve and act via postsynaptic μ-, δ- and -opioid receptors. Treatment of neonatal rat AMC cultures for ∼1 week with μ- and/or δ- (but not ) opioid agonists (2 μm) led to a marked suppression of both hypoxia and hypercapnia sensitivity, as measured by K(+) current inhibition and membrane depolarization; co-incubation with naloxone prevented the effects of combined opioids. The suppression of hypoxia sensitivity was attributable to upregulation of K(ATP) current density and the K(ATP) channel subunit Kir6.2, and was reversed by the K(ATP) channel blocker, glibenclamide. By contrast, suppression of hypercapnia sensitivity was associated with down-regulation of two key mediators of CO(2) sensing, i.e. carbonic anhydrase I and II. Collectively, these studies point to a novel role for opioid receptor signalling in the developmental regulation of chromaffin cell chemosensitivity, and suggest that prenatal exposure to opioid drugs could lead to impaired arousal responses in the neonate.
Figures

 ∼ 15 mmHg) typically causes inhibition of outward K+ current (top trace) and membrane depolarization (bottom trace); current density (pA/pF) versus voltage (I–V) plot (middle) shows significant inhibition of outward current (P < 0.05) at potentials positive to +10 mV. These responses are markedly reduced or abolished in neonatal AMCs chronically exposed for ∼1 week to either a combination of μ-, δ- and κ-opioid agonists (2 μ
∼ 15 mmHg) typically causes inhibition of outward K+ current (top trace) and membrane depolarization (bottom trace); current density (pA/pF) versus voltage (I–V) plot (middle) shows significant inhibition of outward current (P < 0.05) at potentials positive to +10 mV. These responses are markedly reduced or abolished in neonatal AMCs chronically exposed for ∼1 week to either a combination of μ-, δ- and κ-opioid agonists (2 μ



Similar articles
-
Chronic opioids regulate KATP channel subunit Kir6.2 and carbonic anhydrase I and II expression in rat adrenal chromaffin cells via HIF-2α and protein kinase A.Am J Physiol Cell Physiol. 2014 Aug 1;307(3):C266-77. doi: 10.1152/ajpcell.00135.2014. Epub 2014 Jun 4. Am J Physiol Cell Physiol. 2014. PMID: 24898587 Free PMC article.
-
Chronic nicotine blunts hypoxic sensitivity in perinatal rat adrenal chromaffin cells via upregulation of KATP channels: role of alpha7 nicotinic acetylcholine receptor and hypoxia-inducible factor-2alpha.J Neurosci. 2009 Jun 3;29(22):7137-47. doi: 10.1523/JNEUROSCI.0544-09.2009. J Neurosci. 2009. PMID: 19494136 Free PMC article.
-
Ontogeny of O2 and CO2//H+ chemosensitivity in adrenal chromaffin cells: role of innervation.J Exp Biol. 2014 Mar 1;217(Pt 5):673-81. doi: 10.1242/jeb.086165. J Exp Biol. 2014. PMID: 24574383 Review.
-
Chronic nicotine in utero selectively suppresses hypoxic sensitivity in neonatal rat adrenal chromaffin cells.FASEB J. 2008 May;22(5):1317-26. doi: 10.1096/fj.07-9194com. Epub 2007 Dec 10. FASEB J. 2008. PMID: 18070822
-
Regulation of oxygen sensitivity in adrenal chromaffin cells.Ann N Y Acad Sci. 2009 Oct;1177:132-9. doi: 10.1111/j.1749-6632.2009.05031.x. Ann N Y Acad Sci. 2009. PMID: 19845615 Review.
Cited by
-
New insights into carbonic anhydrase inhibition, vasodilation, and treatment of hypertensive-related diseases.Curr Hypertens Rep. 2014 Sep;16(9):467. doi: 10.1007/s11906-014-0467-3. Curr Hypertens Rep. 2014. PMID: 25079851 Review.
-
Gambierol Blocks a K+ Current Fraction without Affecting Catecholamine Release in Rat Fetal Adrenomedullary Cultured Chromaffin Cells.Toxins (Basel). 2022 Apr 2;14(4):254. doi: 10.3390/toxins14040254. Toxins (Basel). 2022. PMID: 35448863 Free PMC article.
-
Innervated adrenomedullary microphysiological system to model nicotine and opioid exposure.Organs Chip. 2021 Nov;3:100009. doi: 10.1016/j.ooc.2021.100009. Epub 2021 Oct 22. Organs Chip. 2021. PMID: 38650595 Free PMC article.
-
Clinical Therapeutic Effect of Naloxone Combined with Hemodialysis on Acute Severe Alcoholism.Med Sci Monit. 2018 Aug 2;24:5363-5367. doi: 10.12659/MSM.908382. Med Sci Monit. 2018. PMID: 30068901 Free PMC article.
-
Fast inactivation of Nav1.3 channels by FGF14 proteins: An unconventional way to regulate the slow firing of adrenal chromaffin cells.J Gen Physiol. 2021 May 3;153(5):e202112879. doi: 10.1085/jgp.202112879. J Gen Physiol. 2021. PMID: 33792614 Free PMC article.
References
-
- Bournaud R, Hidalgo J, Yu H, Girard E, Shimahara T. Catecholamine secretion from rat foetal adrenal chromaffin cells and hypoxia sensitivity. Pflugers Arch. 2007;454:83–92. - PubMed
-
- Burns L, Conroy E, Mattick R. Infant mortality among women on a methadone program during pregnancy. Drug Alcohol Rev. 2010;29:551–556. - PubMed
-
- Buttigieg J, Brown S, Zhang M, Lowe M, Holloway AC, Nurse CA. Chronic nicotine in utero selectively suppresses hypoxic sensitivity in neonatal rat adrenal chromaffin cells. FASEB J. 2007;22:1317–1326. - PubMed
-
- Castanas E, Bourhim N, Giraud P, Boudouresque F, Cantau P, Oliver C. Interaction of opiates with opioid binding sites in the bovine adrenal medulla: I. Interaction with delta and mu sites. J Neurochem. 1985a;45:677–687. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

