Failure of action potential propagation in sensory neurons: mechanisms and loss of afferent filtering in C-type units after painful nerve injury
- PMID: 23148321
- PMCID: PMC3591718
- DOI: 10.1113/jphysiol.2012.242750
Failure of action potential propagation in sensory neurons: mechanisms and loss of afferent filtering in C-type units after painful nerve injury
Abstract
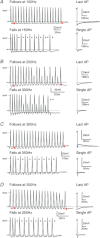
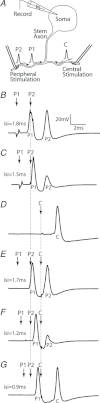
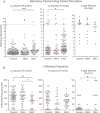
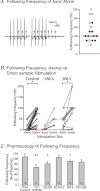
The T-junction of sensory neurons in the dorsal root ganglion (DRG) is a potential impediment to action potential (AP) propagation towards the CNS. Using intracellular recordings from rat DRG neuronal somata during stimulation of the dorsal root, we determined that the maximal rate at which all of 20 APs in a train could successfully transit the T-junction (following frequency) was lowest in C-type units, followed by A-type units with inflected descending limbs of the AP, and highest in A-type units without inflections. In C-type units, following frequency was slower than the rate at which AP trains could be produced in either dorsal root axonal segments or in the soma alone, indicating that the T-junction is a site that acts as a low-pass filter for AP propagation. Following frequency was slower for a train of 20 APs than for two, indicating that a cumulative process leads to propagation failure. Propagation failure was accompanied by diminished somatic membrane input resistance, and was enhanced when Ca(2+)-sensitive K(+) currents were augmented or when Ca(2+)-sensitive Cl(-) currents were blocked. After peripheral nerve injury, following frequencies were increased in axotomized C-type neurons and decreased in axotomized non-inflected A-type neurons. These findings reveal that the T-junction in sensory neurons is a regulator of afferent impulse traffic. Diminished filtering of AP trains at the T-junction of C-type neurons with axotomized peripheral processes could enhance the transmission of activity that is ectopically triggered in a neuroma or the neuronal soma, possibly contributing to pain generation.
Figures





Similar articles
-
Upregulation of T-type Ca2+ channels in primary sensory neurons in spinal nerve injury.Spine (Phila Pa 1976). 2013 Mar 15;38(6):463-70. doi: 10.1097/BRS.0b013e318272fbf8. Spine (Phila Pa 1976). 2013. PMID: 22972512
-
Involvement of hyperpolarization-activated, cyclic nucleotide-gated cation channels in dorsal root ganglion in neuropathic pain.Sheng Li Xue Bao. 2008 Oct 25;60(5):579-80. Sheng Li Xue Bao. 2008. PMID: 18958363
-
Cell type-specific changes of the membrane properties of peripherally-axotomized dorsal root ganglion neurons in a rat model of neuropathic pain.Neuroscience. 1998 Sep;86(1):301-9. doi: 10.1016/s0306-4522(98)00022-0. Neuroscience. 1998. PMID: 9692763
-
Role of decreased sensory neuron membrane calcium currents in the genesis of neuropathic pain.Croat Med J. 2007 Feb;48(1):9-21. Croat Med J. 2007. PMID: 17309135 Free PMC article. Review.
-
Mechanisms of Dorsal Root Ganglion Stimulation in Pain Suppression: A Computational Modeling Analysis.Neuromodulation. 2018 Apr;21(3):234-246. doi: 10.1111/ner.12754. Epub 2018 Jan 29. Neuromodulation. 2018. PMID: 29377442 Review.
Cited by
-
Specifically formulated ketogenic, low carbohydrate, and carnivore diets can prevent migraine: a perspective.Front Nutr. 2024 Apr 30;11:1367570. doi: 10.3389/fnut.2024.1367570. eCollection 2024. Front Nutr. 2024. PMID: 38746934 Free PMC article.
-
Genomic Action of Sigma-1 Receptor Chaperone Relates to Neuropathic Pain.Mol Neurobiol. 2021 Jun;58(6):2523-2541. doi: 10.1007/s12035-020-02276-8. Epub 2021 Jan 18. Mol Neurobiol. 2021. PMID: 33459966 Free PMC article.
-
Local GABAergic signaling within sensory ganglia controls peripheral nociceptive transmission.J Clin Invest. 2017 May 1;127(5):1741-1756. doi: 10.1172/JCI86812. Epub 2017 Apr 4. J Clin Invest. 2017. PMID: 28375159 Free PMC article.
-
Dorsal root ganglia control nociceptive input to the central nervous system.PLoS Biol. 2023 Jan 5;21(1):e3001958. doi: 10.1371/journal.pbio.3001958. eCollection 2023 Jan. PLoS Biol. 2023. PMID: 36603052 Free PMC article.
-
Encoding Taste: From Receptors to Perception.Handb Exp Pharmacol. 2022;275:53-90. doi: 10.1007/164_2021_559. Handb Exp Pharmacol. 2022. PMID: 34796381 Free PMC article.
References
-
- Abdulla FA, Smith PA. Axotomy- and autotomy-induced changes in Ca2+ and K+ channel currents of rat dorsal root ganglion neurons. J Neurophysiol. 2001;85:644–658. - PubMed
-
- Bessou P, Burgess PR, Perl ER, Taylor CB. Dynamic properties of mechanoreceptors with unmyelinated (C) fibers. J Neurophysiol. 1971;34:116–131. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

