A randomised efficacy and discontinuation study of etanercept versus adalimumab (RED SEA) for rheumatoid arthritis: a pragmatic, unblinded, non-inferiority study of first TNF inhibitor use: outcomes over 2 years
- PMID: 23148339
- PMCID: PMC3532970
- DOI: 10.1136/bmjopen-2012-001395
A randomised efficacy and discontinuation study of etanercept versus adalimumab (RED SEA) for rheumatoid arthritis: a pragmatic, unblinded, non-inferiority study of first TNF inhibitor use: outcomes over 2 years
Abstract
Objective: To compare adalimumab versus etanercept in patients with active rheumatoid arthritis (RA) to test the hypothesis that adalimumab was not inferior to etanercept in terms of drug continuation by a margin of 15% after 52 weeks of treatment.
Design: Pragmatic, randomised, parallel group, multicentre, unblinded and non-inferiority trial. Randomisation stratified by baseline use of methotrexate.
Participants: 125 adults with active RA despite treatment with two disease-modifying drugs (DMARDs), including methotrexate randomised (1 : 1) to adalimumab 40 mg alternate weeks or etanercept 50 mg weekly, added to existing medication.
Measurements: The primary outcome was proportion of patients continuing treatment after 52 weeks. Secondary outcomes included: disease activity score using 28 joints (DAS28), treatment satisfaction (TSQM V.2), health status (Euroqol-5D), drug toxicity and persistence with therapy after 2 years.
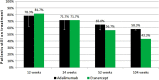
Results: Persistence with therapy was 65% for adalimumab versus 56.7% for etanercept (one-sided 95% CI for proportion still taking adalimumab minus proportion on etanercept ≥-7.9%); demonstrating non-inferiority at the 15% margin. After 2 years these figures were: adalimumab 58.3% and etanecept 43.3% (CI ≥-1.7%). The proportion of good, moderate and non-responders based on DAS28-C reactive protein, after 52 weeks, were 26.3%, 33.3% and 40.4%, respectively, for adalimumab versus 16.7%, 31.7% and 51.7%, respectively, for etanercept (p=0.158). Baseline median EQ-5D scores improved from 0.52 to 0.69 for adalimumab and from 0.52 to 0.64 for etanercept (p=0.046) after 52 weeks. Global satisfaction, effectiveness, side effects and convenience scores based on the TSQM were similar for both drugs. Fourteen serious adverse events occurred including two deaths from myocardial infarction, one patient with ovarian cancer and one with acute myeloid leukaemia.
Conclusions: Clinicians choosing a first tumour necrosis factor inhibitor for active RA, despite trying two DMARDs including methotrexate, may choose either adalimumab or etanercept in the knowledge that these drugs are similarly effective.
Clinical trial registration number: EU Clinical Trials Register 2006-006275-21/GB.
Figures

References
-
- American College of Rheumatology Rheumatoid Arthritis Clinical Trial Investigators Ad Hoc Task Force American College of Rheumatology Clinical Trial Priorities and Design Conference, July 22–23, 2010. Arthritis Rheum 2011;63:2151–6 - PubMed
-
- O'Dell JR. It is the best of times; it is the worst of times: is there a way forward? A plethora of treatment options for rheumatoid arthritis, but critical trial design issue. Arthritis Rheum 2007;56:3884–6 - PubMed
-
- Goekoop-Ruiterman YPM, De Vries-Bouwstra JK, Allaart CF, et al. Comparison of treatment strategies in early rheumatoid arthritis. A randomized trial. Ann Intern Med 2007;146:406–15 - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
