Histone demethylase GASC1--a potential prognostic and predictive marker in invasive breast cancer
- PMID: 23148692
- PMCID: PMC3547738
- DOI: 10.1186/1471-2407-12-516
Histone demethylase GASC1--a potential prognostic and predictive marker in invasive breast cancer
Abstract
Background: The histone demethylase GASC1 (JMJD2C) is an epigenetic factor suspected of involvement in development of different cancers, including breast cancer. It is thought to be overexpressed in the more aggressive breast cancer types based on mRNA expression studies on cell lines and meta analysis of human breast cancer sets. This study aimed to evaluate the prognostic and predictive value of GASC1 for women with invasive breast cancer.
Methods: All the 355 cases were selected from a cohort enrolled in the Kuopio Breast Cancer Project between April 1990 and December 1995. The expression of GASC1 was studied by immunohistochemistry (IHC) on tissue microarrays. Additionally relative GASC1 mRNA expression was measured from available 57 cases.
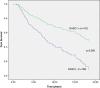
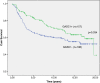
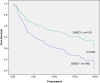
Results: In our material, 56% of the cases were GASC1 negative and 44% positive in IHC staining. Women with GASC1 negative tumors had two years shorter breast cancer specific survival and time to relapse than the women with GASC1 positive tumors (p=0.017 and p=0.034 respectively). The majority of GASC1 negative tumors were ductal cases (72%) of higher histological grade (84% of grade II and III altogether). When we evaluated estrogen receptor negative and progesterone receptor negative cases separately, there was 2 times more GASC1 negative than GASC1 positive tumors in each group (chi2, p= 0.033 and 0.001 respectively). In the HER2 positive cases, there was 3 times more GASC1 negative cases than GASC1 positives (chi2, p= 0.029). Patients treated with radiotherapy (n=206) and hormonal treatment (n=62) had better breast cancer specific survival, when they were GASC1 positive (Cox regression: HR=0.49, p=0.007 and HR=0.33, p=0.015, respectively). The expression of GASC1 mRNA was in agreement with the protein analysis.
Conclusions: This study indicates that the GASC1 is both a prognostic and a predictive factor for women with invasive breast cancer. GASC1 negativity is associated with tumors of more aggressive histopathological types (ductal type, grade II and III, ER negative, PR negative). Patients with GASC1 positive tumors have better breast cancer specific survival and respond better to radiotherapy and hormonal treatment.
Figures





References
-
- Rodriguez-Paredes M, Esteller M. Cancer epigenetics reaches mainstream oncology. Nat Med. 2011;17(3):330–339. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

