A randomized trial of 7-day doripenem versus 10-day imipenem-cilastatin for ventilator-associated pneumonia
- PMID: 23148736
- PMCID: PMC3672596
- DOI: 10.1186/cc11862
A randomized trial of 7-day doripenem versus 10-day imipenem-cilastatin for ventilator-associated pneumonia
Abstract
Introduction: The aim of this study was to compare a 7-day course of doripenem to a 10-day course of imipenem-cilastatin for ventilator-associated pneumonia (VAP) due to Gram-negative bacteria.
Methods: This was a prospective, double-blinded, randomized trial comparing a fixed 7-day course of doripenem one gram as a four-hour infusion every eight hours with a fixed 10-day course of imipenem-cilastatin one gram as a one-hour infusion every eight hours (April 2008 through June 2011).
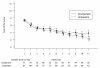
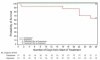
Results: The study was stopped prematurely at the recommendation of the Independent Data Monitoring Committee that was blinded to treatment arm assignment and performed a scheduled review of data which showed signals that were close to the pre-specified stopping limits. The final analyses included 274 randomized patients. The clinical cure rate at the end of therapy (EOT) in the microbiological intent-to-treat (MITT) population was numerically lower for patients in the doripenem arm compared to the imipenem-cilastatin arm (45.6% versus 56.8%; 95% CI, -26.3% to 3.8%). Similarly, the clinical cure rate at EOT was numerically lower for patients with Pseudomonas aeruginosa VAP, the most common Gram-negative pathogen, in the doripenem arm compared to the imipenem-cilastatin arm (41.2% versus 60.0%; 95% CI, -57.2 to 19.5). All cause 28-day mortality in the MITT group was numerically greater for patients in the doripenem arm compared to the imipenem-cilastatin arm (21.5% versus 14.8%; 95% CI, -5.0 to 18.5) and for patients with P. aeruginosa VAP (35.3% versus 0.0%; 95% CI, 12.6 to 58.0).
Conclusions: Among patients with microbiologically confirmed late-onset VAP, a fixed 7-day course of doripenem was found to have non-significant higher rates of clinical failure and mortality compared to a fixed 10-day course of imipenem-cilastatin. Consideration should be given to treating patients with VAP for more than seven days to optimize clinical outcome.
Trial registration: ClinicalTrials.gov: NCT00589693.
Figures

Comment in
-
A week seems to be weak: tailoring duration of antibiotic treatment in Gram-negative ventilator-associated pneumonia.Crit Care. 2013 Jan 21;17(1):106. doi: 10.1186/cc11899. Crit Care. 2013. PMID: 23336300 Free PMC article.
References
-
- Vincent JL, Rello J, Marshall J, Silva E, Anzueto A, Martin CD, Moreno R, Lipman J, Gomersall C, Sakr Y, Reinhart K. EPIC II Group of Investigators. International study of the prevalence and outcomes of infection in intensive care units. JAMA. 2009;16:2323–2329. doi: 10.1001/jama.2009.1754. - DOI - PubMed
-
- American Thoracic Society Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;16:388–416. - PubMed
-
- Chastre J, Wolff M, Fagon JY, Chevret S, Thomas F, Wermert D, Clementi E, Gonzalez J, Jusserand D, Asfar P, Perrin D, Fieux F, Aubas S. PneumA Trial Group. Comparison of 8 vs 15 days of antibiotic therapy for ventilator-associated, pneumonia in adults: a randomized trial. JAMA. 2003;16:2588–2598. doi: 10.1001/jama.290.19.2588. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

