Human conditions of insulin-like growth factor-I (IGF-I) deficiency
- PMID: 23148873
- PMCID: PMC3543345
- DOI: 10.1186/1479-5876-10-224
Human conditions of insulin-like growth factor-I (IGF-I) deficiency
Abstract
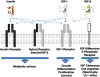
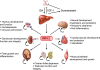
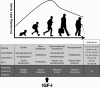
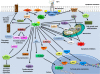
Insulin-like growth factor I (IGF-I) is a polypeptide hormone produced mainly by the liver in response to the endocrine GH stimulus, but it is also secreted by multiple tissues for autocrine/paracrine purposes. IGF-I is partly responsible for systemic GH activities although it possesses a wide number of own properties (anabolic, antioxidant, anti-inflammatory and cytoprotective actions). IGF-I is a closely regulated hormone. Consequently, its logical therapeutical applications seems to be limited to restore physiological circulating levels in order to recover the clinical consequences of IGF-I deficiency, conditions where, despite continuous discrepancies, IGF-I treatment has never been related to oncogenesis. Currently the best characterized conditions of IGF-I deficiency are Laron Syndrome, in children; liver cirrhosis, in adults; aging including age-related-cardiovascular and neurological diseases; and more recently, intrauterine growth restriction. The aim of this review is to summarize the increasing list of roles of IGF-I, both in physiological and pathological conditions, underlying that its potential therapeutical options seem to be limited to those proven states of local or systemic IGF-I deficiency as a replacement treatment, rather than increasing its level upper the normal range.
Figures
Similar articles
-
An experimental model of partial insulin-like growth factor-1 deficiency in mice.J Physiol Biochem. 2014 Mar;70(1):129-39. doi: 10.1007/s13105-013-0287-y. Epub 2013 Sep 18. J Physiol Biochem. 2014. PMID: 24043429
-
[Insulin-like growth factor I (IGF-I) and liver cirrhosis].Rev Esp Enferm Dig. 2007 Mar;99(3):156-64. doi: 10.4321/s1130-01082007000300007. Rev Esp Enferm Dig. 2007. PMID: 17516829 Review. Spanish.
-
Insulin-like growth factor-I deficiency in children with growth hormone insensitivity: current and future treatment options.BioDrugs. 2009;23(3):155-63. doi: 10.2165/00063030-200923030-00002. BioDrugs. 2009. PMID: 19627167 Review.
-
Insulin-like growth factor-I and the liver.Liver Int. 2011 Aug;31(7):911-9. doi: 10.1111/j.1478-3231.2010.02428.x. Epub 2011 Jan 24. Liver Int. 2011. PMID: 21733081 Review.
-
Intrauterine Growth Retardation (IUGR) as a Novel Condition of Insulin-Like Growth Factor-1 (IGF-1) Deficiency.Rev Physiol Biochem Pharmacol. 2016;170:1-35. doi: 10.1007/112_2015_5001. Rev Physiol Biochem Pharmacol. 2016. PMID: 26634242 Review.
Cited by
-
Organ-Size Regulation in Mammals.Cold Spring Harb Perspect Biol. 2015 Jul 17;7(9):a019240. doi: 10.1101/cshperspect.a019240. Cold Spring Harb Perspect Biol. 2015. PMID: 26187729 Free PMC article. Review.
-
Histopathologic evaluation of the inflammatory factors and stromal cells in the endometriosis lesions: A case-control study.Int J Reprod Biomed. 2022 Nov 2;20(10):819-830. doi: 10.18502/ijrm.v20i10.12266. eCollection 2022 Oct. Int J Reprod Biomed. 2022. PMID: 36381357 Free PMC article.
-
Structure of the PAPP-ABP5 complex reveals mechanism of substrate recognition.Nat Commun. 2022 Sep 20;13(1):5500. doi: 10.1038/s41467-022-33175-2. Nat Commun. 2022. PMID: 36127359 Free PMC article.
-
Type I insulin-like growth factor as a liver reserve assessment tool in hepatocellular carcinoma.J Hepatocell Carcinoma. 2015 Sep 18;2:131-42. doi: 10.2147/JHC.S81309. eCollection 2015. J Hepatocell Carcinoma. 2015. PMID: 27508202 Free PMC article. Review.
-
SM22α (Smooth Muscle Protein 22-α) Promoter-Driven IGF1R (Insulin-Like Growth Factor 1 Receptor) Deficiency Promotes Atherosclerosis.Arterioscler Thromb Vasc Biol. 2018 Oct;38(10):2306-2317. doi: 10.1161/ATVBAHA.118.311134. Arterioscler Thromb Vasc Biol. 2018. PMID: 30354209 Free PMC article.
References
-
- Salmon WD Jr, Daughaday WH. A hormonally controlled serum factor which stimulates sulfate incorporation by cartilage in vitro. J Lab Clin Med. 1957;49(6):825–836. - PubMed
-
- Froesch ER, Buergi H, Ramseier EB, Bally P, Labhart A. Antibody-suppressible and nonsuppressible insulin-like activities in human serum and their physiologic significance. An insulin assay with adipose tissue of increased precision and specificity. J Clin Invest. 1963;42:1816–1834. doi: 10.1172/JCI104866. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

