Intravenous HOE-642 reduces brain edema and Na uptake in the rat permanent middle cerebral artery occlusion model of stroke: evidence for participation of the blood-brain barrier Na/H exchanger
- PMID: 23149557
- PMCID: PMC3564192
- DOI: 10.1038/jcbfm.2012.160
Intravenous HOE-642 reduces brain edema and Na uptake in the rat permanent middle cerebral artery occlusion model of stroke: evidence for participation of the blood-brain barrier Na/H exchanger
Abstract
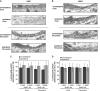
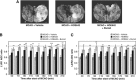
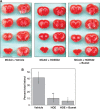
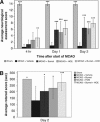
Cerebral edema forms in the early hours of ischemic stroke by processes involving increased transport of Na and Cl from blood into brain across an intact blood-brain barrier (BBB). Our previous studies provided evidence that the BBB Na-K-Cl cotransporter is stimulated by the ischemic factors hypoxia, aglycemia, and arginine vasopressin (AVP), and that inhibition of the cotransporter by intravenous bumetanide greatly reduces edema and infarct in rats subjected to permanent middle cerebral artery occlusion (pMCAO). More recently, we showed that BBB Na/H exchanger activity is also stimulated by hypoxia, aglycemia, and AVP. The present study was conducted to further investigate the possibility that a BBB Na/H exchanger also participates in edema formation during ischemic stroke. Sprague-Dawley rats were subjected to pMCAO and then brain edema and Na content assessed by magnetic resonance imaging diffusion-weighed imaging and magnetic resonance spectroscopy Na spectroscopy, respectively, for up to 210 minutes. We found that intravenous administration of the specific Na/H exchange inhibitor HOE-642 significantly decreased brain Na uptake and reduced cerebral edema, brain swelling, and infarct volume. These findings support the hypothesis that edema formation and brain Na uptake during the early hours of cerebral ischemia involve BBB Na/H exchanger activity as well as Na-K-Cl cotransporter activity.
Figures

Similar articles
-
Effects of estradiol on ischemic factor-induced astrocyte swelling and AQP4 protein abundance.Am J Physiol Cell Physiol. 2011 Jul;301(1):C204-12. doi: 10.1152/ajpcell.00399.2010. Epub 2011 Apr 6. Am J Physiol Cell Physiol. 2011. PMID: 21471464 Free PMC article.
-
Bumetanide inhibition of the blood-brain barrier Na-K-Cl cotransporter reduces edema formation in the rat middle cerebral artery occlusion model of stroke.J Cereb Blood Flow Metab. 2004 Sep;24(9):1046-56. doi: 10.1097/01.WCB.0000130867.32663.90. J Cereb Blood Flow Metab. 2004. PMID: 15356425
-
Cerebral microvascular endothelial cell Na/H exchange: evidence for the presence of NHE1 and NHE2 isoforms and regulation by arginine vasopressin.Am J Physiol Cell Physiol. 2009 Aug;297(2):C278-89. doi: 10.1152/ajpcell.00093.2009. Epub 2009 May 20. Am J Physiol Cell Physiol. 2009. PMID: 19458287 Free PMC article.
-
Blood-brain barrier Na transporters in ischemic stroke.Adv Pharmacol. 2014;71:113-46. doi: 10.1016/bs.apha.2014.06.011. Epub 2014 Aug 23. Adv Pharmacol. 2014. PMID: 25307215 Review.
-
Sodium ion/hydrogen ion exchange inhibition: a new pharmacologic approach to myocardial ischemia and reperfusion injury.J Clin Pharmacol. 1998 Oct;38(10):887-97. doi: 10.1002/j.1552-4604.1998.tb04383.x. J Clin Pharmacol. 1998. PMID: 9807968 Review.
Cited by
-
In vivo detection of acute intracellular acidification in glioblastoma multiforme following a single dose of cariporide.Int J Clin Oncol. 2018 Oct;23(5):812-819. doi: 10.1007/s10147-018-1289-0. Epub 2018 May 10. Int J Clin Oncol. 2018. PMID: 29749579
-
Inflammatory resolution and vascular barrier restoration after retinal ischemia reperfusion injury.J Neuroinflammation. 2021 Aug 26;18(1):186. doi: 10.1186/s12974-021-02237-5. J Neuroinflammation. 2021. PMID: 34446062 Free PMC article.
-
Endothelial Na+/H+ exchanger NHE1 participates in redox-sensitive leukocyte recruitment triggered by methylglyoxal.Cardiovasc Diabetol. 2014 Sep 30;13:134. doi: 10.1186/s12933-014-0134-7. Cardiovasc Diabetol. 2014. PMID: 25270604 Free PMC article.
-
Hemorrhagic Stroke Induces a Time-Dependent Upregulation of miR-150-5p and miR-181b-5p in the Bloodstream.Front Neurol. 2021 Oct 27;12:736474. doi: 10.3389/fneur.2021.736474. eCollection 2021. Front Neurol. 2021. PMID: 34777204 Free PMC article.
-
HOE-642 improves the protection of hypothermia on neuronal mitochondria after cardiac arrest in rats.Am J Transl Res. 2020 May 15;12(5):2181-2191. eCollection 2020. Am J Transl Res. 2020. PMID: 32509210 Free PMC article.
References
-
- Betz AL. Alterations in cerebral endothelial cell function in ischemia. Adv Neurol. 1996;71:301–313. - PubMed
-
- Foroutan S, Brillault J, Forbush B, O'Donnell ME. Moderate-to-severe ischemic conditions increase activity and phosphorylation of the cerebral microvascular endothelial cell Na+-K+-Cl- cotransporter. Am J Physiol Cell Physiol. 2005;289:C1492–C1501. - PubMed
-
- O'Donnell ME, Tran L, Lam TI, Liu XB, Anderson SE. Bumetanide inhibition of the blood-brain barrier Na-K-Cl cotransporter reduces edema formation in the rat middle cerebral artery occlusion model of stroke. J Cereb Blood Flow Metab. 2004;24:1046–1056. - PubMed
-
- Brillault J, Lam TI, Rutkowsky JM, Foroutan S, O'Donnell ME. Hypoxia effects on cell volume and ion uptake of cerebral microvascular endothelial cells. Am J Physiol Cell Physiol. 2008;294:C88–C96. - PubMed
-
- O'Donnell ME, Lam TI, Tran LQ, Foroutan S, Anderson SE. Estradiol reduces activity of the blood-brain barrier Na-K-Cl cotransporter and decreases edema formation in permanent middle cerebral artery occlusion. J Cereb Blood Flow Metab. 2006;26:1234–1249. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

