Detection of systemic and mucosal HPV-specific IgG and IgA antibodies in adolescent girls one and two years after HPV vaccination
- PMID: 23149693
- PMCID: PMC3859753
- DOI: 10.4161/hv.22693
Detection of systemic and mucosal HPV-specific IgG and IgA antibodies in adolescent girls one and two years after HPV vaccination
Abstract
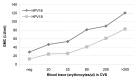
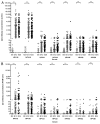
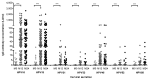
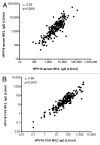
The bivalent HPV16/18 vaccine induces high antibody concentrations in serum while data about antibody responses in the cervix are limited. In this study, we investigated pre- and post-vaccination antibody responses against seven high-risk HPV types by detection of IgG and IgA HPV-specific antibodies in cervical secretion samples (CVS) and serum. From an HPV vaccine monitoring study CVS and serum samples were available (pre-vaccination (n = 297), one year (n = 211) and two years (n = 141) post-dose-one vaccination) from girls aged 14-16 y. The girls were vaccinated with the bivalent HPV vaccine at months 0, 1 and 6. CVS was self-sampled using a tampon. Samples were tested for HPV-specific antibodies (HPV16/18/31/33/45/52/58) by a VLP-based multiplex immunoassay. Post-vaccination, IgG and IgA antibody levels for HPV16/18 were detectable in CVS and amounted to 2% and 1% of the IgG and IgA antibody levels observed in serum, respectively. The antibody levels remained constant between one and two years after vaccination. The correlation between CVS and serum was similar for IgG and IgA vaccine-derived antibody levels for HPV16 (rs = 0.58, rs = 0.54) and HPV18 (rs = 0.50, rs = 0.55). Vaccine-derived IgG antibody levels against cross-reactive HPV types in CVS and in serum were highest for HPV45. No IgA cross-reactive antibody responses could be detected in CVS. Post-vaccination, HPV16/18 IgG and IgA antibodies are not only detectable in serum but also in CVS. The correlation of HPV16/18 IgG antibody levels between serum and CVS suggests that vaccine induced HPV antibodies transudate and/or exudate from the systemic circulation to the cervical mucosa to provide protection against HPV infections.
Keywords: HPV; HPV vaccination; IgA; IgG; antibody concentrations; cervical secretion; exudation; multiplex-immunoassay; transudation.
Figures
References
-
- Schwarz TF, Kocken M, Petäjä T, Einstein MH, Spaczynski M, Louwers JA, et al. Correlation between levels of human papillomavirus (HPV)-16 and 18 antibodies in serum and cervicovaginal secretions in girls and women vaccinated with the HPV-16/18 AS04-adjuvanted vaccine. Hum Vaccin. 2010;6:1054–61. doi: 10.4161/hv.6.12.13399. - DOI - PubMed
-
- Lehtinen M, Paavonen J, Wheeler CM, Jaisamrarn U, Garland SM, Castellsagué X, et al. HPV PATRICIA Study Group Overall efficacy of HPV-16/18 AS04-adjuvanted vaccine against grade 3 or greater cervical intraepithelial neoplasia: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol. 2012;13:89–99. - PubMed
-
- Muñoz N, Manalastas R, Jr., Pitisuttithum P, Tresukosol D, Monsonego J, Ault K, et al. Safety, immunogenicity, and efficacy of quadrivalent human papillomavirus (types 6, 11, 16, 18) recombinant vaccine in women aged 24-45 years: a randomised, double-blind trial. Lancet. 2009;373:1949–57. doi: 10.1016/S0140-6736(09)60691-7. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
