A peptide derived from laminin-γ3 reversibly impairs spermatogenesis in rats
- PMID: 23149730
- PMCID: PMC3538133
- DOI: 10.1038/ncomms2171
A peptide derived from laminin-γ3 reversibly impairs spermatogenesis in rats
Abstract
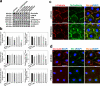
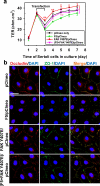
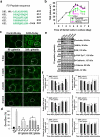
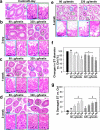
Cellular events that occur across the seminiferous epithelium in the mammalian testis during spermatogenesis are tightly coordinated by biologically active peptides released from laminin chains. Laminin-γ3 domain IV is released at the apical ectoplasmic specialization during spermiation and mediates restructuring of the blood-testis barrier, which facilitates the transit of preleptotene spermatocytes. Here we determine the biologically active domain in laminin-γ3 domain IV, which we designate F5 peptide, and show that the overexpression of this domain, or the use of a synthetic F5 peptide, in Sertoli cells with an established functional blood-testis barrier reversibly perturbs blood-testis barrier integrity in vitro and in the rat testis in vivo. This effect is mediated via changes in protein distribution at the Sertoli and Sertoli-germ-cell cell interface and by phosphorylation of focal adhesion kinase at Tyr(407). The consequences are perturbed organization of actin filaments in Sertoli cells, disruption of the blood-testis barrier and spermatid loss. The impairment of spermatogenesis suggests that this laminin peptide fragment may serve as a contraceptive in male rats.
Figures

Similar articles
-
An autocrine axis in the testis that coordinates spermiation and blood-testis barrier restructuring during spermatogenesis.Proc Natl Acad Sci U S A. 2008 Jul 1;105(26):8950-5. doi: 10.1073/pnas.0711264105. Epub 2008 Jun 25. Proc Natl Acad Sci U S A. 2008. PMID: 18579774 Free PMC article.
-
F5-peptide induces aspermatogenesis by disrupting organization of actin- and microtubule-based cytoskeletons in the testis.Oncotarget. 2016 Sep 27;7(39):64203-64220. doi: 10.18632/oncotarget.11887. Oncotarget. 2016. PMID: 27611949 Free PMC article.
-
c-Yes regulates cell adhesion at the blood-testis barrier and the apical ectoplasmic specialization in the seminiferous epithelium of rat testes.Int J Biochem Cell Biol. 2011 Apr;43(4):651-65. doi: 10.1016/j.biocel.2011.01.008. Epub 2011 Jan 21. Int J Biochem Cell Biol. 2011. PMID: 21256972 Free PMC article.
-
A local regulatory network in the testis mediated by laminin and collagen fragments that supports spermatogenesis.Crit Rev Biochem Mol Biol. 2021 Jun;56(3):236-254. doi: 10.1080/10409238.2021.1901255. Epub 2021 Mar 25. Crit Rev Biochem Mol Biol. 2021. PMID: 33761828 Review.
-
Intercellular adhesion molecules (ICAMs) and spermatogenesis.Hum Reprod Update. 2013 Mar-Apr;19(2):167-86. doi: 10.1093/humupd/dms049. Epub 2013 Jan 3. Hum Reprod Update. 2013. PMID: 23287428 Free PMC article. Review.
Cited by
-
NC1 domain of collagen α3(IV) derived from the basement membrane regulates Sertoli cell blood-testis barrier dynamics.Spermatogenesis. 2013 Apr 1;3(2):e25465. doi: 10.4161/spmg.25465. Spermatogenesis. 2013. PMID: 23885308 Free PMC article.
-
Basement Membrane Laminin α2 Regulation of BTB Dynamics via Its Effects on F-Actin and Microtubule Cytoskeletons Is Mediated Through mTORC1 Signaling.Endocrinology. 2017 Apr 1;158(4):963-978. doi: 10.1210/en.2016-1630. Endocrinology. 2017. PMID: 28323988 Free PMC article.
-
Cdc42 is involved in NC1 peptide-regulated BTB dynamics through actin and microtubule cytoskeletal reorganization.FASEB J. 2019 Dec;33(12):14461-14478. doi: 10.1096/fj.201900991R. Epub 2019 Nov 2. FASEB J. 2019. PMID: 31682474 Free PMC article.
-
Breast cancer resistance protein regulates apical ectoplasmic specialization dynamics stage specifically in the rat testis.Am J Physiol Endocrinol Metab. 2013 Apr 1;304(7):E757-69. doi: 10.1152/ajpendo.00645.2012. Epub 2013 Feb 12. Am J Physiol Endocrinol Metab. 2013. PMID: 23403943 Free PMC article.
-
rpS6 regulates blood-testis barrier dynamics through Arp3-mediated actin microfilament organization in rat sertoli cells. An in vitro study.Endocrinology. 2015 May;156(5):1900-13. doi: 10.1210/en.2014-1791. Epub 2015 Feb 25. Endocrinology. 2015. PMID: 25714812 Free PMC article.
References
-
- de Kretser DM, Kerr JB. The cytology of the testis. In: Knobil E, et al., editors. The Physiology of Reproduction. Vol. 1. Raven Press; New York: 1988. pp. 837–932.
-
- Sharpe RM. Regulation of spermatogenesis. In: Knobil E, Neill JD, editors. The Physiology of Reproduction. Raven Press; New York: 1994. pp. 1363–1434.
-
- Parvinen M. Regulation of the seminiferous epithelium. Endocr Rev. 1982;3:404–417. - PubMed
-
- Hess RA, de Franca LR. Spermatogenesis and cycle of the seminiferous epithelium. Adv Exp Med Biol. 2008;636:1–15. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

