Structural modelling and mutant cycle analysis predict pharmacoresponsiveness of a Na(V)1.7 mutant channel
- PMID: 23149731
- PMCID: PMC3530897
- DOI: 10.1038/ncomms2184
Structural modelling and mutant cycle analysis predict pharmacoresponsiveness of a Na(V)1.7 mutant channel
Abstract
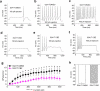
Sodium channel Na(V)1.7 is critical for human pain signalling. Gain-of-function mutations produce pain syndromes including inherited erythromelalgia, which is usually resistant to pharmacotherapy, but carbamazepine normalizes activation of Na(V)1.7-V400M mutant channels from a family with carbamazepine-responsive inherited erythromelalgia. Here we show that structural modelling and thermodynamic analysis predict pharmacoresponsiveness of another mutant channel (S241T) that is located 159 amino acids distant from V400M. Structural modelling reveals that Na(v)1.7-S241T is ~2.4 Å apart from V400M in the folded channel, and thermodynamic analysis demonstrates energetic coupling of V400M and S241T during activation. Atomic proximity and energetic coupling are paralleled by pharmacological coupling, as carbamazepine (30 μM) depolarizes S214T activation, as previously reported for V400M. Pharmacoresponsiveness of S241T to carbamazepine was further evident at a cellular level, where carbamazepine normalized the hyperexcitability of dorsal root ganglion neurons expressing S241T. We suggest that this approach might identify variants that confer enhanced pharmacoresponsiveness on a variety of channels.
Figures

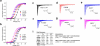




Similar articles
-
Reverse pharmacogenomics: carbamazepine normalizes activation and attenuates thermal hyperexcitability of sensory neurons due to Nav 1.7 mutation I234T.Br J Pharmacol. 2018 Jun;175(12):2261-2271. doi: 10.1111/bph.13935. Epub 2017 Jul 30. Br J Pharmacol. 2018. PMID: 28658526 Free PMC article.
-
Pharmacotherapy for Pain in a Family With Inherited Erythromelalgia Guided by Genomic Analysis and Functional Profiling.JAMA Neurol. 2016 Jun 1;73(6):659-67. doi: 10.1001/jamaneurol.2016.0389. JAMA Neurol. 2016. PMID: 27088781
-
Nav1.7-A1632G Mutation from a Family with Inherited Erythromelalgia: Enhanced Firing of Dorsal Root Ganglia Neurons Evoked by Thermal Stimuli.J Neurosci. 2016 Jul 13;36(28):7511-22. doi: 10.1523/JNEUROSCI.0462-16.2016. J Neurosci. 2016. PMID: 27413160 Free PMC article.
-
The Na(V)1.7 sodium channel: from molecule to man.Nat Rev Neurosci. 2013 Jan;14(1):49-62. doi: 10.1038/nrn3404. Epub 2012 Dec 12. Nat Rev Neurosci. 2013. PMID: 23232607 Review.
-
Familial pain syndromes from mutations of the NaV1.7 sodium channel.Ann N Y Acad Sci. 2010 Jan;1184:196-207. doi: 10.1111/j.1749-6632.2009.05110.x. Ann N Y Acad Sci. 2010. PMID: 20146699 Review.
Cited by
-
Molecular architecture of a sodium channel S6 helix: radial tuning of the voltage-gated sodium channel 1.7 activation gate.J Biol Chem. 2013 May 10;288(19):13741-7. doi: 10.1074/jbc.M113.462366. Epub 2013 Mar 27. J Biol Chem. 2013. PMID: 23536180 Free PMC article.
-
SCN10A Mutation in a Patient with Erythromelalgia Enhances C-Fiber Activity Dependent Slowing.PLoS One. 2016 Sep 6;11(9):e0161789. doi: 10.1371/journal.pone.0161789. eCollection 2016. PLoS One. 2016. PMID: 27598514 Free PMC article.
-
Peripheral Voltage-Gated Cation Channels in Neuropathic Pain and Their Potential as Therapeutic Targets.Front Pain Res (Lausanne). 2021 Dec 13;2:750583. doi: 10.3389/fpain.2021.750583. eCollection 2021. Front Pain Res (Lausanne). 2021. PMID: 35295464 Free PMC article. Review.
-
Stem cell-derived sensory neurons modelling inherited erythromelalgia: normalization of excitability.Brain. 2023 Jan 5;146(1):359-371. doi: 10.1093/brain/awac031. Brain. 2023. PMID: 35088838 Free PMC article.
-
Network topology of NaV1.7 mutations in sodium channel-related painful disorders.BMC Syst Biol. 2017 Feb 24;11(1):28. doi: 10.1186/s12918-016-0382-0. BMC Syst Biol. 2017. PMID: 28235406 Free PMC article.
References
-
- Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington (DC): 2011. - PubMed
-
- Waxman SG. Neurobiology: a channel sets the gain on pain. Nature. 2006;444:831–832. - PubMed
-
- Dib-Hajj SD, Cummins TR, Black JA, Waxman SG. From genes to pain: Na v 1.7 and human pain disorders. Trends Neurosci. 2007;30:555–563. - PubMed
-
- Dib-Hajj SD, Cummins TR, Black JA, Waxman SG. Sodium channels in normal and pathological pain. Annual review of neuroscience. 2010;33:325–347. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

