Accuracy of lung nodule density on HRCT: analysis by PSF-based image simulation
- PMID: 23149779
- PMCID: PMC5718548
- DOI: 10.1120/jacmp.v13i6.3868
Accuracy of lung nodule density on HRCT: analysis by PSF-based image simulation
Abstract
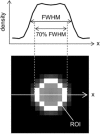
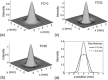
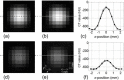
A computed tomography (CT) image simulation technique based on the point spread function (PSF) was applied to analyze the accuracy of CT-based clinical evaluations of lung nodule density. The PSF of the CT system was measured and used to perform the lung nodule image simulation. Then, the simulated image was resampled at intervals equal to the pixel size and the slice interval found in clinical high-resolution CT (HRCT) images. On those images, the nodule density was measured by placing a region of interest (ROI) commonly used for routine clinical practice, and comparing the measured value with the true value (a known density of object function used in the image simulation). It was quantitatively determined that the measured nodule density depended on the nodule diameter and the image reconstruction parameters (kernel and slice thickness). In addition, the measured density fluctuated, depending on the offset between the nodule center and the image voxel center. This fluctuation was reduced by decreasing the slice interval (i.e., with the use of overlapping reconstruction), leading to a stable density evaluation. Our proposed method of PSF-based image simulation accompanied with resampling enables a quantitative analysis of the accuracy of CT-based evaluations of lung nodule density. These results could potentially reveal clinical misreadings in diagnosis, and lead to more accurate and precise density evaluations. They would also be of value for determining the optimum scan and reconstruction parameters, such as image reconstruction kernels and slice thicknesses/intervals.
Figures








References
-
- Sone S, Matsumoto T, Honda T, et al. HRCT features of small peripheral lung carcinomas detected in a low‐dose CT screening program. Acad Radiol. 2010;17(1):75–83. - PubMed
-
- Sone S, Tsushima K, Yoshida K, Hamanaka K, Hanaoka T, Kondo R. Pulmonary nodules: preliminary experience with semiautomated volumetric evaluation by CT stratum. Acad Radiol. 2010;17(7):900–11. - PubMed
-
- Iwano S, Nakamura T, Kamioka Y, Ikeda M, Ishigaki T. Computer‐aided differentiation of malignant from benign solitary pulmonary nodules imaged by high‐resolution CT. Comput Med Imaging Graph. 2008;32(5):416–22. - PubMed
-
- Iwano S, Makino N, Ikeda M, et al. Solitary pulmonary nodules: optimal slice thickness of high‐resolution CT in differentiating malignant from benign. Clin Imaging. 2004;28(5):322–28. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

