Outcome and pathologic classification of children and adolescents with mediastinal large B-cell lymphoma treated with FAB/LMB96 mature B-NHL therapy
- PMID: 23149845
- PMCID: PMC3544113
- DOI: 10.1182/blood-2012-04-422709
Outcome and pathologic classification of children and adolescents with mediastinal large B-cell lymphoma treated with FAB/LMB96 mature B-NHL therapy
Abstract
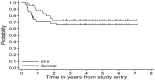
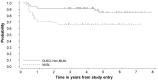
Mediastinal large B-cell lymphoma (MLBL) represents 2% of mature B-cell non-Hodgkin lymphoma in patients ≤ 18 years of age. We analyzed data from childhood and adolescent patients with stage III MLBL (n = 42) and non-MLBL DLBCL (n = 69) treated with Group B therapy in the French-American-British/Lymphome Malins de Burkitt (FAB/LMB) 96 study. MLBL patients had a male/female 26/16; median age, 15.7 years (range, 12.5-19.7); and LDH < 2 versus ≥ 2 × the upper limit of normal, 23:19. Six MLBL patients (14%) had < a 20% response to initial COP (cyclophosphamide, vincristine, and prednisone) therapy. Central pathology revealed approximately 50% with classical features of primary MLBL. Five-year event-free survival for the stage III MLBL and non-MLBL DLBCL groups was 66% (95% confidence interval [CI], 49%-78%) and 85% (95% CI, 71%-92%), respectively (P < .001; 14%). The 5-year overall survival in the 42 MLBL patients was 73% (95% CI, 56%-84%). We conclude that MLBL in adolescent patients is associated with significantly inferior event-free survival compared with stage III non-MLBL DLBCL and can be of multiple histologies. Alternate treatment strategies should be investigated in the future taking into account both adult MLBL approaches and more recent biologic findings in adult MLBL.
Figures

Comment in
-
Pediatric MLBL: challenges remain.Blood. 2013 Jan 10;121(2):245-6. doi: 10.1182/blood-2012-11-468629. Blood. 2013. PMID: 23307971
Similar articles
-
Pediatric MLBL: challenges remain.Blood. 2013 Jan 10;121(2):245-6. doi: 10.1182/blood-2012-11-468629. Blood. 2013. PMID: 23307971
-
Outcomes of adults and children with primary mediastinal B-cell lymphoma treated with dose-adjusted EPOCH-R.Br J Haematol. 2017 Dec;179(5):739-747. doi: 10.1111/bjh.14951. Epub 2017 Oct 29. Br J Haematol. 2017. PMID: 29082519 Free PMC article.
-
[Eight-year experience in treating aggressive mediastinal large B-cell lymphomas].Ter Arkh. 2013;85(7):50-6. Ter Arkh. 2013. PMID: 24137947 Russian.
-
Primary Mediastinal B-Cell Lymphoma in Children and Young Adults.J Natl Compr Canc Netw. 2023 Mar;21(3):323-330. doi: 10.6004/jnccn.2023.7004. J Natl Compr Canc Netw. 2023. PMID: 36898366 Review.
-
Primary mediastinal large B-cell lymphoma: the need for prospective controlled clinical trials.Leuk Lymphoma. 1999 Nov;35(5-6):537-44. doi: 10.1080/10428199909169618. Leuk Lymphoma. 1999. PMID: 10609791 Review.
Cited by
-
Ruxolitinib significantly enhances in vitro apoptosis in Hodgkin lymphoma and primary mediastinal B-cell lymphoma and survival in a lymphoma xenograft murine model.Oncotarget. 2018 Jan 18;9(11):9776-9788. doi: 10.18632/oncotarget.24267. eCollection 2018 Feb 9. Oncotarget. 2018. PMID: 29515770 Free PMC article.
-
Dose-adjusted EPOCH-rituximab or intensified B-NHL therapy for pediatric primary mediastinal large B-cell lymphoma.Haematologica. 2021 Dec 1;106(12):3232-3235. doi: 10.3324/haematol.2021.278971. Haematologica. 2021. PMID: 34498443 Free PMC article. No abstract available.
-
Excellent outcome of children/adolescents with primary mediastinal large B-cell lymphoma treated with a FAB/ LMB-based chemotherapy regimen with rituximab.Haematologica. 2024 Nov 1;109(11):3790-3794. doi: 10.3324/haematol.2024.285403. Haematologica. 2024. PMID: 38988265 Free PMC article. No abstract available.
-
Obinutuzumab (GA101) vs. rituximab significantly enhances cell death, antibody-dependent cytotoxicity and improves overall survival against CD20+ primary mediastinal B-cell lymphoma (PMBL) in a xenograft NOD-scid IL2Rgnull (NSG) mouse model: a potential targeted agent in the treatment of PMBL.Oncotarget. 2020 Aug 11;11(32):3035-3047. doi: 10.18632/oncotarget.27691. eCollection 2020 Aug 11. Oncotarget. 2020. PMID: 32850008 Free PMC article.
-
International Pediatric Non-Hodgkin Lymphoma Response Criteria.J Clin Oncol. 2015 Jun 20;33(18):2106-11. doi: 10.1200/JCO.2014.59.0745. Epub 2015 May 4. J Clin Oncol. 2015. PMID: 25940725 Free PMC article.
References
-
- Jaffe ES, Harris NL, Stein H, Vardiman JW, editors. Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues. WHO Classification of Tumours. Vol 3. Lyon, France: International Agency for Research on Cancer; 2001.
-
- Swerdlow S, Campo E, Harris NL, editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissue. Geneva, Switzerland: World Health Organization; 2008. International Agency for Research on Cancer. pp. 1–439.
-
- Murphy SB. Classification, staging and end results of treatment of childhood non-Hodgkin's lymphomas: dissimilarities from lymphomas in adults. Semin Oncol. 1980;7(3):332–339. - PubMed
-
- Lones MA, Perkins SL, Sposto R, et al. Large-cell lymphoma arising in the mediastinum in children and adolescents is associated with an excellent outcome: a Children's Cancer Group report. J Clin Oncol. 2000;18(22):3845–3853. - PubMed
-
- Seidemann K, Tiemann M, Lauterbach I, et al. Primary mediastinal large B-cell lymphoma with sclerosis in pediatric and adolescent patients: treatment and results from three therapeutic studies of the Berlin-Frankfurt-Munster Group. J Clin Oncol. 2003;21(9):1782–1789. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

