Tasquinimod Is an Allosteric Modulator of HDAC4 survival signaling within the compromised cancer microenvironment
- PMID: 23149916
- PMCID: PMC3578133
- DOI: 10.1158/0008-5472.CAN-12-2730
Tasquinimod Is an Allosteric Modulator of HDAC4 survival signaling within the compromised cancer microenvironment
Abstract
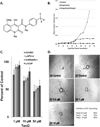
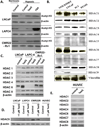
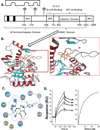
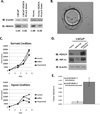
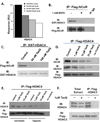
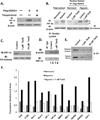
Tasquinimod is an orally active antiangiogenic drug that is currently in phase III clinical trials for the treatment of castration-resistant prostate cancer. However, the target of this drug has remained unclear. In this study, we applied diverse strategies to identify the histone deacetylase HDAC4 as a target for the antiangiogenic activity of tasquinimod. Our comprehensive analysis revealed allosteric binding (Kd 10-30 nmol/L) to the regulatory Zn(2+) binding domain of HDAC4 that locks the protein in a conformation preventing HDAC4/N-CoR/HDAC3 complex formation. This binding inhibited colocalization of N-CoR/HDAC3, thereby inhibiting deacetylation of histones and HDAC4 client transcription factors, such as HIF-1α, which are bound at promoter/enhancers where epigenetic reprogramming is required for cancer cell survival and angiogenic response. Through this mechanism, tasquinimod is effective as a monotherapeutic agent against human prostate, breast, bladder, and colon tumor xenografts, where its efficacy could be further enhanced in combination with a targeted thapsigargin prodrug (G202) that selectively kills tumor endothelial cells. Together, our findings define a mechanism of action of tasquinimod and offer a perspective on how its clinical activity might be leveraged in combination with other drugs that target the tumor microenvironment. Cancer Res; 73(4); 1386-99. ©2012 AACR.
Figures

References
-
- Pili R, Haggman M, Stadler W, Gingrich JR, Assikis VJ, Bjork A, et al. A randomized multicenter international phase II study of tasquinimod in chemotherapy-naive patients with metastatic castration-resistant prostate cancer. J Clin Oncol. 2010;28:75.
-
- Isaacs JT, Pili R, Qian DZ, Dalrymple SL, Garrison JB, Kyprianou N, et al. Identification of ABR-215050 as lead second generation quinoline-3-carboxamide anti-angiogenic agent for the treatment of prostate cancer. Prostate. 2006;66:1768–1778. - PubMed
-
- Dalrymple SL, Becker RE, Isaacs JT. The quinoline-3-carboxamide anti-angiogenic agent, tasquinimod, enhances the anti-prostate cancer efficacy of androgen ablation and taxotere without effecting serum PSA directly in human xenografts. Prostate. 2007;67:790–797. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

