A quantitative signaling screen identifies CARD11 mutations in the CARD and LATCH domains that induce Bcl10 ubiquitination and human lymphoma cell survival
- PMID: 23149938
- PMCID: PMC3554118
- DOI: 10.1128/MCB.00850-12
A quantitative signaling screen identifies CARD11 mutations in the CARD and LATCH domains that induce Bcl10 ubiquitination and human lymphoma cell survival
Abstract
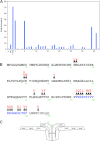
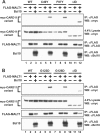
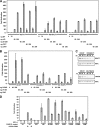
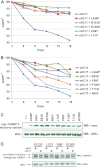
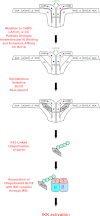
Antigen receptor signaling to NF-κB, essential for normal lymphocyte activation, is dysregulated in several types of lymphoma. During normal signaling, the multidomain adapter CARD11 transitions from a closed, inactive state to an open, active scaffold that assembles a multiprotein complex, leading to NF-κB activation. The regulation of CARD11 scaffold function is bypassed by lymphoma-associated oncogenic CARD11 mutations that induce spontaneous signaling. We report an unbiased high-throughput quantitative signaling screen that identifies new CARD11 hyperactive variants and defines a LATCH domain that functions with the CARD to promote CARD11 autoinhibition. Gain-of-function mutations in the LATCH or CARD disrupt inhibitory domain binding, promote Bcl10 association, and induce Bcl10 ubiquitination, NF-κB activation, and human lymphoma cell survival. Our results identify CARD11 mutations with oncogenic potential, provide a mechanistic explanation for their signaling potency, and offer a straightforward method for the discovery of variants that promote the tumorigenesis of NF-κB-dependent lymphomas.
Figures





Similar articles
-
Molecular Determinants of Scaffold-induced Linear Ubiquitinylation of B Cell Lymphoma/Leukemia 10 (Bcl10) during T Cell Receptor and Oncogenic Caspase Recruitment Domain-containing Protein 11 (CARD11) Signaling.J Biol Chem. 2016 Dec 9;291(50):25921-25936. doi: 10.1074/jbc.M116.754028. Epub 2016 Oct 24. J Biol Chem. 2016. PMID: 27777308 Free PMC article.
-
The protein kinase C-responsive inhibitory domain of CARD11 functions in NF-kappaB activation to regulate the association of multiple signaling cofactors that differentially depend on Bcl10 and MALT1 for association.Mol Cell Biol. 2008 Sep;28(18):5668-86. doi: 10.1128/MCB.00418-08. Epub 2008 Jul 14. Mol Cell Biol. 2008. PMID: 18625728 Free PMC article.
-
Lymphomagenic CARD11/BCL10/MALT1 signaling drives malignant B-cell proliferation via cooperative NF-κB and JNK activation.Proc Natl Acad Sci U S A. 2015 Dec 29;112(52):E7230-8. doi: 10.1073/pnas.1507459112. Epub 2015 Dec 14. Proc Natl Acad Sci U S A. 2015. PMID: 26668357 Free PMC article.
-
Genetic errors of the human caspase recruitment domain-B-cell lymphoma 10-mucosa-associated lymphoid tissue lymphoma-translocation gene 1 (CBM) complex: Molecular, immunologic, and clinical heterogeneity.J Allergy Clin Immunol. 2015 Nov;136(5):1139-49. doi: 10.1016/j.jaci.2015.06.031. Epub 2015 Aug 12. J Allergy Clin Immunol. 2015. PMID: 26277595 Free PMC article. Review.
-
The CARD11-BCL10-MALT1 (CBM) signalosome complex: Stepping into the limelight of human primary immunodeficiency.J Allergy Clin Immunol. 2014 Aug;134(2):276-84. doi: 10.1016/j.jaci.2014.06.015. J Allergy Clin Immunol. 2014. PMID: 25087226 Free PMC article. Review.
Cited by
-
Phase 1b trial of an ibrutinib-based combination therapy in recurrent/refractory CNS lymphoma.Blood. 2019 Jan 31;133(5):436-445. doi: 10.1182/blood-2018-09-875732. Epub 2018 Dec 19. Blood. 2019. PMID: 30567753 Free PMC article. Clinical Trial.
-
QRICH1 mediates an intracellular checkpoint for CD8+ T cell activation via the CARD11 signalosome.Sci Immunol. 2025 Mar 14;10(105):eadn8715. doi: 10.1126/sciimmunol.adn8715. Epub 2025 Mar 14. Sci Immunol. 2025. PMID: 40085689 Free PMC article.
-
BCL10-CARD11 Fusion Mimics an Active CARD11 Seed That Triggers Constitutive BCL10 Oligomerization and Lymphocyte Activation.Front Immunol. 2018 Nov 20;9:2695. doi: 10.3389/fimmu.2018.02695. eCollection 2018. Front Immunol. 2018. PMID: 30515170 Free PMC article.
-
A Novel, Heterozygous Three Base-Pair Deletion in CARD11 Results in B Cell Expansion with NF-κB and T Cell Anergy Disease.J Clin Immunol. 2020 Feb;40(2):406-411. doi: 10.1007/s10875-019-00729-x. Epub 2020 Jan 2. J Clin Immunol. 2020. PMID: 31897776
-
BCL10 Mutations Define Distinct Dependencies Guiding Precision Therapy for DLBCL.Cancer Discov. 2022 Aug 5;12(8):1922-1941. doi: 10.1158/2159-8290.CD-21-1566. Cancer Discov. 2022. PMID: 35658124 Free PMC article.
References
-
- Schulze-Luehrmann J, Ghosh S. 2006. Antigen-receptor signaling to nuclear factor kappa B. Immunity 25: 701–715 - PubMed
-
- Kaileh M, Sen R. 2012. NF-kappaB function in B lymphocytes. Immunol. Rev. 246: 254–271 - PubMed
-
- Staudt LM. 2010. Oncogenic activation of NF-kappaB. Cold Spring Harb. Perspect. Biol. 2: a000109 doi:10.1101/cshperspect.a000109 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
