Efficacy and safety of novel oral anticoagulants for treatment of acute venous thromboembolism: direct and adjusted indirect meta-analysis of randomised controlled trials
- PMID: 23150473
- PMCID: PMC3496553
- DOI: 10.1136/bmj.e7498
Efficacy and safety of novel oral anticoagulants for treatment of acute venous thromboembolism: direct and adjusted indirect meta-analysis of randomised controlled trials
Abstract
Objective: To critically review the effectiveness of the novel oral anticoagulants (rivaroxaban, dabigatran, ximelagatran, and apixaban) in the treatment of acute venous thromboembolism.
Design: Systematic review and random effects meta-analysis. Data were extracted independently by two investigators. An adjusted indirect comparison was performed to compare between novel oral anticoagulants.
Data sources: Medline, Embase, and Cochrane Library (from inception to April 2012). Hand searching of relevant scientific works and contact with experts.
Study selection: Randomised controlled trials of novel oral anticoagulants compared with vitamin K antagonists for acute venous thromboembolism. Selected outcomes were recurrent events, major bleeding, and all cause mortality.
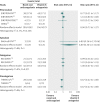
Results: Nine studies met our inclusion criteria, involving 16,701 patients evaluated for efficacy and 16,611 for safety. Data were stratified according to different novel oral anticoagulants. For recurrent acute venous thromboembolism, there were no significant differences in events rates between any of the anticoagulants and conventional treatment (rivaroxaban (four studies): relative risk 0.85, 95% confidence interval 0.55 to 1.31; dabigatran (two studies): 1.09, 0.76 to 1.57; ximelagatran (two studies): 1.06, 0.62 to 1.80; and apixaban (one study): 0.98, 0.20 to 4.79). Rivaroxaban reduced the risk of major bleeding compared with conventional treatment (0.57, 0.39 to 0.84), whereas other novel oral anticoagulants did not (0.76 (0.49 to 1.18) for dabigatran; 0.54 (0.28 to 1.03) for ximelagatran; 2.95 (0.12 to 71.82) for apixaban). For all cause mortality there were no significant differences between the novel oral anticoagulants and conventional treatment (0.96 (0.72 to 1.27) for rivaroxaban; 1.00 (0.67 to 1.50) for dabigatran; 0.67 (0.42 to 1.08) for ximelagatran; 6.89 (0.36 to 132.06) for apixaban). The adjusted indirect comparison between rivaroxaban and dabigatran did not show superiority of either drug over the others for major bleeding (0.75, 0.41 to 1.34) or the other endpoints.
Conclusions: Compared with vitamin K antagonists, the novel oral anticoagulants had a similar risk of recurrence of acute venous thromboembolism and all cause mortality, though rivaroxaban was associated with a reduced risk of bleeding.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at
Figures



References
-
- Kearon C, Akl EA, Comerota AJ, Prandoni P, Bounameaux H, Goldhaber SZ, et al. Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012;141:e419-94S. - PMC - PubMed
-
- Weitz JI. New oral anticoagulants: a view from the laboratory. Am J Hematol 2012;S1:S133-6. - PubMed
-
- Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions 5.1.0. Cochrane Collaboration, 2011.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
