Implantable, multifunctional, bioresorbable optics
- PMID: 23150544
- PMCID: PMC3511733
- DOI: 10.1073/pnas.1209056109
Implantable, multifunctional, bioresorbable optics
Abstract
Advances in personalized medicine are symbiotic with the development of novel technologies for biomedical devices. We present an approach that combines enhanced imaging of malignancies, therapeutics, and feedback about therapeutics in a single implantable, biocompatible, and resorbable device. This confluence of form and function is accomplished by capitalizing on the unique properties of silk proteins as a mechanically robust, biocompatible, optically clear biomaterial matrix that can house, stabilize, and retain the function of therapeutic components. By developing a form of high-quality microstructured optical elements, improved imaging of malignancies and of treatment monitoring can be achieved. The results demonstrate a unique family of devices for in vitro and in vivo use that provide functional biomaterials with built-in optical signal and contrast enhancement, demonstrated here with simultaneous drug delivery and feedback about drug delivery with no adverse biological effects, all while slowly degrading to regenerate native tissue.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
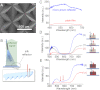
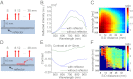
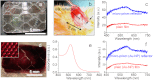
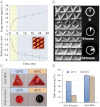
References
-
- Howard D, et al. Immunoselection and adenoviral genetic modulation of human osteoprogenitors: In vivo bone formation on PLA scaffold. Biochem Biophys Res Commun. 2002;299(2):208–215. - PubMed
-
- Partridge K, et al. Adenoviral BMP-2 gene transfer in mesenchymal stem cells: In vitro and in vivo bone formation on biodegradable polymer scaffolds. Biochem Biophys Res Commun. 2002;292(1):144–152. - PubMed
-
- Stone KR, Steadman JR, Rodkey WG, Li ST. Regeneration of meniscal cartilage with use of a collagen scaffold. Analysis of preliminary data. J Bone Joint Surg Am. 1997;79A(12):1770–1777. - PubMed
-
- Parker ST, et al. Biocompatible silk printed optical waveguides. Adv Mater. 2009;21(23):2411–2415.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

