FoxO is a critical regulator of stem cell maintenance in immortal Hydra
- PMID: 23150562
- PMCID: PMC3511741
- DOI: 10.1073/pnas.1209714109
FoxO is a critical regulator of stem cell maintenance in immortal Hydra
Erratum in
- Proc Natl Acad Sci U S A. 2013 Jan 8;110(2):797
Abstract
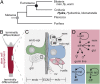
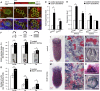
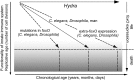
Hydra's unlimited life span has long attracted attention from natural scientists. The reason for that phenomenon is the indefinite self-renewal capacity of its stem cells. The underlying molecular mechanisms have yet to be explored. Here, by comparing the transcriptomes of Hydra's stem cells followed by functional analysis using transgenic polyps, we identified the transcription factor forkhead box O (FoxO) as one of the critical drivers of this continuous self-renewal. foxO overexpression increased interstitial stem cell and progenitor cell proliferation and activated stem cell genes in terminally differentiated somatic cells. foxO down-regulation led to an increase in the number of terminally differentiated cells, resulting in a drastically reduced population growth rate. In addition, it caused down-regulation of stem cell genes and antimicrobial peptide (AMP) expression. These findings contribute to a molecular understanding of Hydra's immortality, indicate an evolutionarily conserved role of FoxO in controlling longevity from Hydra to humans, and have implications for understanding cellular aging.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Martínez DE. Mortality patterns suggest lack of senescence in hydra. Exp Gerontol. 1998;33(3):217–225. - PubMed
-
- Bosch TC. Hydra and the evolution of stem cells. Bioessays. 2009;31(4):478–486. - PubMed
-
- Khalturin K, et al. Transgenic stem cells in Hydra reveal an early evolutionary origin for key elements controlling self-renewal and differentiation. Dev Biol. 2007;309(1):32–44. - PubMed
-
- Bosch TC, Anton-Erxleben F, Hemmrich G, Khalturin K. The Hydra polyp: Nothing but an active stem cell community. Dev Growth Differ. 2010;52(1):15–25. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Medical

