Activation of liver X receptor induces macrophage interleukin-5 expression
- PMID: 23150660
- PMCID: PMC3527921
- DOI: 10.1074/jbc.M112.403394
Activation of liver X receptor induces macrophage interleukin-5 expression
Abstract
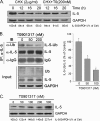
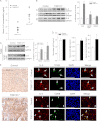
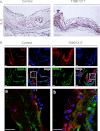
IL-5 stimulates production of T15/EO6 IgM antibodies that can block the uptake of oxidized low density lipoprotein by macrophages, whereas a deficiency in macrophage IL-5 expression accelerates development of atherosclerosis. Liver X receptors (LXRs) are ligand-activated transcription factors that can induce macrophage ABCA1 expression and cholesterol efflux, thereby inhibiting the development of atherosclerosis. However, it remains unknown whether additional mechanisms, such as the regulation of macrophage IL-5 expression, are related to the anti-atherogenic properties of LXR. We initially defined IL-5 expression in macrophages where the LXR ligand (T0901317) induced macrophage IL-5 protein expression and secretion. The overexpression of LXR increased, whereas its knockdown inhibited IL-5 expression. Furthermore, we found that LXR activation increased IL-5 transcripts, promoter activity, formation of an LXR·LXR-responsive element complex, and IL-5 protein stability. In vivo, we found that T0901317 increased IL-5 and total IgM levels in plasma and IL-5 expression in multiple tissues in wild type mice. In LDL receptor knock-out (LDLR(-/-)) mice, T0901317 increased IL-5 expression in the aortic root area. Taken together, our studies demonstrate that macrophage IL-5 is a target gene for LXR activation, and the induction of macrophage IL-5 expression can be related to LXR-inhibited atherosclerosis.
Figures



Similar articles
-
Activation of liver X receptor decreases atherosclerosis in Ldlr⁻/⁻ mice in the absence of ATP-binding cassette transporters A1 and G1 in myeloid cells.Arterioscler Thromb Vasc Biol. 2014 Feb;34(2):279-84. doi: 10.1161/ATVBAHA.113.302781. Epub 2013 Dec 5. Arterioscler Thromb Vasc Biol. 2014. PMID: 24311381 Free PMC article.
-
Liver X receptor agonist methyl-3β-hydroxy-5α,6α-epoxycholanate attenuates atherosclerosis in apolipoprotein E knockout mice without increasing plasma triglyceride.Pharmacology. 2010;86(5-6):306-12. doi: 10.1159/000321320. Epub 2010 Nov 10. Pharmacology. 2010. PMID: 21071998
-
The effect of T0901317 on ATP-binding cassette transporter A1 and Niemann-Pick type C1 in apoE-/- mice.J Cardiovasc Pharmacol. 2008 May;51(5):467-75. doi: 10.1097/FJC.0b013e31816a5be3. J Cardiovasc Pharmacol. 2008. PMID: 18437096
-
Liver X receptor: a potential target in the treatment of atherosclerosis.Expert Opin Ther Targets. 2022 Jul;26(7):645-658. doi: 10.1080/14728222.2022.2117610. Epub 2022 Sep 5. Expert Opin Ther Targets. 2022. PMID: 36003057 Review.
-
Liver x receptor signaling pathways and atherosclerosis.Arterioscler Thromb Vasc Biol. 2010 Aug;30(8):1513-8. doi: 10.1161/ATVBAHA.109.191197. Arterioscler Thromb Vasc Biol. 2010. PMID: 20631351 Free PMC article. Review.
Cited by
-
Peroxisome Proliferator-Activated Receptor-Gamma Reduces ER Stress and Inflammation via Targeting NGBR Expression.Front Pharmacol. 2022 Jan 17;12:817784. doi: 10.3389/fphar.2021.817784. eCollection 2021. Front Pharmacol. 2022. PMID: 35111067 Free PMC article.
-
LongShengZhi Capsule Attenuates Alzheimer-Like Pathology in APP/PS1 Double Transgenic Mice by Reducing Neuronal Oxidative Stress and Inflammation.Front Aging Neurosci. 2020 Nov 23;12:582455. doi: 10.3389/fnagi.2020.582455. eCollection 2020. Front Aging Neurosci. 2020. PMID: 33328962 Free PMC article.
-
Activation of CTU2 expression by LXR promotes the development of hepatocellular carcinoma.Cell Biol Toxicol. 2024 Apr 17;40(1):23. doi: 10.1007/s10565-024-09862-9. Cell Biol Toxicol. 2024. PMID: 38630355 Free PMC article.
-
Activation of Peroxisome Proliferator-activated Receptor γ (PPARγ) and CD36 Protein Expression: THE DUAL PATHOPHYSIOLOGICAL ROLES OF PROGESTERONE.J Biol Chem. 2016 Jul 15;291(29):15108-18. doi: 10.1074/jbc.M116.726737. Epub 2016 May 12. J Biol Chem. 2016. PMID: 27226602 Free PMC article.
-
MEK1/2 inhibitor inhibits neointima formation by activating miR-126-3p/ C-X-C motif chemokine ligand 12 (CXCL12)/C-X-C motif chemokine receptor 4 (CXCR4) axis.Bioengineered. 2022 Apr;13(4):11214-11227. doi: 10.1080/21655979.2022.2063496. Bioengineered. 2022. PMID: 35485167 Free PMC article.
References
-
- Mitra S., Deshmukh A., Sachdeva R., Lu J., Mehta J. L. (2011) Oxidized low-density lipoprotein and atherosclerosis implications in antioxidant therapy. Am. J. Med. Sci. 342, 135–142 - PubMed
-
- Esterbauer H., Schaur R. J., Zollner H. (1991) Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic. Biol. Med. 11, 81–128 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

