Altering hydrophobic sequence lengths shows that hydrophobic mismatch controls affinity for ordered lipid domains (rafts) in the multitransmembrane strand protein perfringolysin O
- PMID: 23150664
- PMCID: PMC3543017
- DOI: 10.1074/jbc.M112.415596
Altering hydrophobic sequence lengths shows that hydrophobic mismatch controls affinity for ordered lipid domains (rafts) in the multitransmembrane strand protein perfringolysin O
Abstract
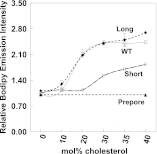
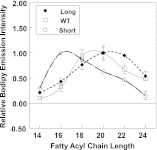
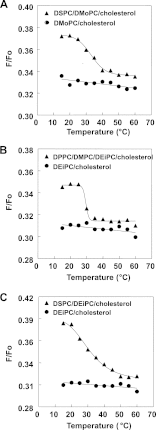
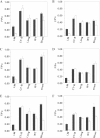
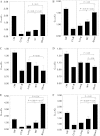
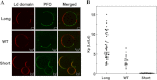
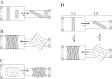
The hypothesis that mismatch between transmembrane (TM) length and bilayer width controls TM protein affinity for ordered lipid domains (rafts) was tested using perfringolysin O (PFO), a pore-forming cholesterol-dependent cytolysin. PFO forms a multimeric barrel with many TM segments. The properties of PFO mutants with lengthened or shortened TM segments were compared with that of PFO with wild type TM sequences. Both mutant and wild type length PFO exhibited cholesterol-dependent membrane insertion. Maximal PFO-induced pore formation occurred in vesicles with wider bilayers for lengthened TM segments and in thinner bilayers for shortened TM segments. In diC(18:0) phosphatidylcholine (PC)/diC(14:1) PC/cholesterol vesicles, which form ordered domains with a relatively thick bilayer and disordered domains with a relatively thin bilayer, affinity for ordered domains was greatest with lengthened TM segments and least with shortened TM segments as judged by FRET. Similar results were observed by microscopy in giant vesicles containing sphingomyelin in place of diC(18:0) PC. In contrast, in diC(16:0) PC/diC(14:0) PC/diC(20:1) PC/cholesterol vesicles, which should form ordered domains with a relatively thin bilayer and disordered domains with a relatively thick bilayer, relative affinity for ordered domains was greatest with shortened TM segments and least with lengthened TM segments. The inability of multi-TM segment proteins (unlike single TM segment proteins) to adapt to mismatch by tilting may explain the sensitivity of raft affinity to mismatch. The difference in width sensitivity for single and multi-TM helix proteins may link raft affinity to multimeric state and thus control the assembly of multimeric TM complexes in rafts.
Figures
References
-
- Simons K., Ikonen E. (1997) Functional rafts in cell membranes. Nature 387, 569–572 - PubMed
-
- Lingwood D., Simons K. (2010) Lipid rafts as a membrane-organizing principle. Science 327, 46–50 - PubMed
-
- Simons K., Toomre D. (2000) Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 1, 31–39 - PubMed
-
- Brown D. A., London E. (1998) Structure and origin of ordered lipid domains in biological membranes. J. Membr. Biol. 164, 103–114 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

