Long-term update of US GI intergroup RTOG 98-11 phase III trial for anal carcinoma: survival, relapse, and colostomy failure with concurrent chemoradiation involving fluorouracil/mitomycin versus fluorouracil/cisplatin
- PMID: 23150707
- PMCID: PMC3515768
- DOI: 10.1200/JCO.2012.43.8085
Long-term update of US GI intergroup RTOG 98-11 phase III trial for anal carcinoma: survival, relapse, and colostomy failure with concurrent chemoradiation involving fluorouracil/mitomycin versus fluorouracil/cisplatin
Abstract
Purpose: On initial publication of GI Intergroup Radiation Therapy Oncology Group (RTOG) 98-11 [A Phase III Randomized Study of 5-Fluorouracil (5-FU), Mitomycin, and Radiotherapy Versus 5-Fluorouracil, Cisplatin and Radiotherapy in Carcinoma of the Anal Canal], concurrent chemoradiation (CCR) with fluorouracil (FU) plus mitomycin (MMC) decreased colostomy failure (CF) when compared with induction plus concurrent FU plus cisplatin (CDDP), but did not significantly impact disease-free survival (DFS) or overall survival (OS) for anal canal carcinoma. The intent of the updated analysis was to determine the long-term impact of treatment on survival (DFS, OS, colostomy-free survival [CFS]), CF, and relapse (locoregional failure [LRF], distant metastasis) in this patient group.
Patients and methods: Stratification factors included sex, clinical node status, and primary size. DFS and OS were estimated univariately by the Kaplan-Meier method, and treatment arms were compared by log-rank test. Time to relapse and CF were estimated by the cumulative incidence method and treatment arms were compared by using Gray's test. Multivariate analyses used Cox proportional hazard models to test for treatment differences after adjusting for stratification factors.
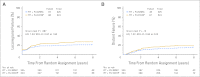
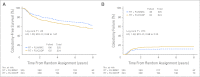
Results: Of 682 patients accrued, 649 were analyzable for outcomes. DFS and OS were statistically better for RT + FU/MMC versus RT + FU/CDDP (5-year DFS, 67.8% v 57.8%; P = .006; 5-year OS, 78.3% v 70.7%; P = .026). There was a trend toward statistical significance for CFS (P = .05), LRF (P = .087), and CF (P = .074). Multivariate analysis was statistically significant for treatment and clinical node status for both DFS and OS, for tumor diameter for DFS, and for sex for OS.
Conclusion: CCR with FU/MMC has a statistically significant, clinically meaningful impact on DFS and OS versus induction plus concurrent FU/CDDP, and it has borderline significance for CFS, CF, and LRF. Therefore, RT + FU/MMC remains the preferred standard of care.
Trial registration: ClinicalTrials.gov NCT00003596.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures

References
-
- Nigro ND, Seydel HG, Considine B, et al. Combined preoperative radiation and chemotherapy for squamous cell carcinoma of the anal canal. Cancer. 1983;51:1826–1829. - PubMed
-
- Sischy B, Doggett RL, Krall JM, et al. Definitive irradiation and chemotherapy for radiosensitization in management of anal carcinoma: Interim report on Radiation Therapy Oncology Group study no. 8314. J Natl Cancer Inst. 1989;81:850–856. - PubMed
-
- Cummings BJ, Keane TJ, O'Sullivan B, et al. Epidermoid anal cancer: Treatment by radiation alone or by radiation and 5-fluorouracil with and without mitomycin C. Int J Radiat Oncol Biol Phys. 1991;21:1115–1125. - PubMed
-
- Flam M, John M, Pajak TF, et al. Role of mitomycin in combination with fluorouracil and radiotherapy, and salvage chemoradiation in the definitive nonsurgical treatment of epidermoid carcinoma of the anal canal: Results of a phase III randomized intergroup study. J Clin Oncol. 1996;14:2527–2539. - PubMed
-
- [No authors listed]: Epidermoid anal cancer: Results from the UKCCCR randomised trial of radiotherapy alone versus radiotherapy, 5-fluorouracil, and mitomycin: UKCCCR Anal Cancer Trial Working Party—UK Co-ordinating Committee on Cancer Research. Lancet. 1996;348:1049–1054. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

