Dual Roles of MDM2 in the Regulation of p53: Ubiquitination Dependent and Ubiquitination Independent Mechanisms of MDM2 Repression of p53 Activity
- PMID: 23150757
- PMCID: PMC3494363
- DOI: 10.1177/1947601912455199
Dual Roles of MDM2 in the Regulation of p53: Ubiquitination Dependent and Ubiquitination Independent Mechanisms of MDM2 Repression of p53 Activity
Abstract
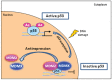
MDM2 oncogenic protein is the principal cellular antagonist of the p53 tumor suppresser gene. p53 activity needs exquisite control to elicit appropriate responses to differential cellular stress conditions. p53 becomes stabilized and active upon various types of stresses. However, too much p53 is not beneficial to cells and causes lethality. At the steady state, p53 activity needs to be leashed for cell survival. Early studies suggested that the MDM2 oncoprotein negatively regulates p53 activity through the induction of p53 protein degradation. MDM2 serves as an E3 ubiquitin ligase of p53; it catalyzes polyubiquitination and subsequently induces proteasome degradation to downregulate p53 protein level. However, the mechanism by which MDM2 represses p53 is not a single mode. Emerging evidence reveals another cellular location of MDM2-p53 interaction. MDM2 is recruited to chromatin, specifically the p53 responsive promoter regions, in a p53 dependent manner. MDM2 is proposed to directly inhibit p53 transactivity at chromatin. This article provides an overview of the mechanism by which p53 is repressed by MDM2 in both ubiquitination dependent and ubiquitination independent pathways.
Keywords: MDM2; antirepression model; ubiquitination dependent; ubiquitination independent.
Conflict of interest statement
Figures

Similar articles
-
RNF12 promotes p53-dependent cell growth suppression and apoptosis by targeting MDM2 for destruction.Cancer Lett. 2016 May 28;375(1):133-141. doi: 10.1016/j.canlet.2016.02.013. Epub 2016 Feb 27. Cancer Lett. 2016. PMID: 26926424
-
UBE4B, a ubiquitin chain assembly factor, is required for MDM2-mediated p53 polyubiquitination and degradation.Cell Cycle. 2011 Jun 15;10(12):1912-5. doi: 10.4161/cc.10.12.15882. Epub 2011 Jun 15. Cell Cycle. 2011. PMID: 21558803 Free PMC article. Review.
-
Acetylation-dependent regulation of MDM2 E3 ligase activity dictates its oncogenic function.Sci Signal. 2017 Feb 14;10(466):eaai8026. doi: 10.1126/scisignal.aai8026. Sci Signal. 2017. PMID: 28196907 Free PMC article.
-
The multiple levels of regulation by p53 ubiquitination.Cell Death Differ. 2010 Jan;17(1):86-92. doi: 10.1038/cdd.2009.77. Cell Death Differ. 2010. PMID: 19543236 Free PMC article. Review.
-
Escape, or Vanish: Control the Fate of p53 through MDM2-Mediated Ubiquitination.Anticancer Agents Med Chem. 2015;16(2):174-89. doi: 10.2174/1871520615666150907093358. Anticancer Agents Med Chem. 2015. PMID: 26343143 Review.
Cited by
-
Impacts of Nutlin-3a and exercise on murine double minute 2-enriched glioma treatment.Neural Regen Res. 2025 Apr 1;20(4):1135-1152. doi: 10.4103/NRR.NRR-D-23-00875. Epub 2024 Mar 1. Neural Regen Res. 2025. PMID: 38989952 Free PMC article.
-
Changes in O-Linked N-Acetylglucosamine (O-GlcNAc) Homeostasis Activate the p53 Pathway in Ovarian Cancer Cells.J Biol Chem. 2016 Sep 2;291(36):18897-914. doi: 10.1074/jbc.M116.734533. Epub 2016 Jul 11. J Biol Chem. 2016. PMID: 27402830 Free PMC article.
-
Valproic Acid Exposure of Pregnant Rats During Organogenesis Disturbs Pancreas Development in Insulin Synthesis and Secretion of the Offspring.Toxicol Res. 2018 Apr;34(2):173-182. doi: 10.5487/TR.2018.34.2.173. Epub 2018 Apr 15. Toxicol Res. 2018. PMID: 29686779 Free PMC article.
-
Individual and combined effect of TP53, MDM2, MDM4, MTHFR, CCR5, and CASP8 gene polymorphisms in lung cancer.Oncotarget. 2017 Nov 29;9(3):3214-3229. doi: 10.18632/oncotarget.22756. eCollection 2018 Jan 9. Oncotarget. 2017. PMID: 29423041 Free PMC article.
-
Combining Oncolytic Virotherapy with p53 Tumor Suppressor Gene Therapy.Mol Ther Oncolytics. 2017 Mar 21;5:20-40. doi: 10.1016/j.omto.2017.03.002. eCollection 2017 Jun 16. Mol Ther Oncolytics. 2017. PMID: 28480326 Free PMC article. Review.
References
-
- Lane DP, Crawford LV. T antigen is bound to a host protein in SV40-transformed cells. Nature. 1979;278:261-3 - PubMed
-
- Linzer DI, Levine AJ. Characterization of a 54K dalton cellular SV40 tumor antigen present in SV40-transformed cells and uninfected embryonal carcinoma cells. Cell. 1979;17:43-52 - PubMed
-
- Eliyahu D, Raz A, Gruss P, Givol D, Oren M. Participation of p53 cellular tumour antigen in transformation of normal embryonic cells. Nature. 1984;312:646-9 - PubMed
-
- Jenkins JR, Rudge K, Currie GA. Cellular immortalization by a cDNA clone encoding the transformation-associated phosphoprotein p53. Nature. 1984;312:651-4 - PubMed
-
- Parada LF, Land H, Weinberg RA, Wolf D, Rotter V. Cooperation between gene encoding p53 tumour antigen and ras in cellular transformation. Nature. 1984;312:649-51 - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
