Multivitamin supplementation in HIV infected adults initiating antiretroviral therapy in Uganda: the protocol for a randomized double blinded placebo controlled efficacy trial
- PMID: 23151221
- PMCID: PMC3519743
- DOI: 10.1186/1471-2334-12-304
Multivitamin supplementation in HIV infected adults initiating antiretroviral therapy in Uganda: the protocol for a randomized double blinded placebo controlled efficacy trial
Abstract
Background: Use of multivitamin supplements during the pre-HAART era has been found to reduce viral load, enhance immune response, and generally improve clinical outcomes among HIV-infected adults. However, immune reconstitution is incomplete and significant mortality and opportunistic infections occur in spite of HAART. There is insufficient research information on whether multivitamin supplementation may be beneficial as adjunct therapy for HIV-infected individuals taking HAART. We propose to evaluate the efficacy of a single recommended daily allowance (RDA) of micronutrients (including vitamins B-complex, C, and E) in slowing disease progression among HIV-infected adults receiving HAART in Uganda.
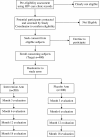
Methods/design: We are using a randomized, double-blind, placebo-controlled trial study design. Eligible patients are HIV-positive adults aged at least 18 years, and are randomized to receive either a placebo; or multivitamins that include a single RDA of the following vitamins: 1.4 mg B1, 1.4 mg B2, 1.9 mg B6, 2.6 mcg B12, 18 mg niacin, 70 mg C, 10 mg E, and 0.4 mg folic acid. Participants are followed for up to 18 months with evaluations at baseline, 6, 12 and 18 months. The study is primarily powered to examine the effects on immune reconstitution, weight gain, and quality of life. In addition, we will examine the effects on other secondary outcomes including the risks of development of new or recurrent disease progression event, including all-cause mortality; ARV regimen change from first- to second-line therapy; and other adverse events as indicated by incident peripheral neuropathy, severe anemia, or diarrhea.
Discussions: The conduct of this trial provides an opportunity to evaluate the potential benefits of this affordable adjunct therapy (multivitamin supplementation) among HIV-infected adults receiving HAART in a developing country setting.
Trial registration: Clinical Trial Registration-URL: http://www.clinicaltrials.gov. Unique identifier: NCT01228578.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical