Clinical trial in healthy malaria-naïve adults to evaluate the safety, tolerability, immunogenicity and efficacy of MuStDO5, a five-gene, sporozoite/hepatic stage Plasmodium falciparum DNA vaccine combined with escalating dose human GM-CSF DNA
- PMID: 23151451
- PMCID: PMC3601132
- DOI: 10.4161/hv.22129
Clinical trial in healthy malaria-naïve adults to evaluate the safety, tolerability, immunogenicity and efficacy of MuStDO5, a five-gene, sporozoite/hepatic stage Plasmodium falciparum DNA vaccine combined with escalating dose human GM-CSF DNA
Abstract
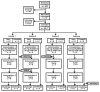
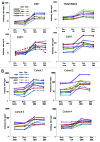
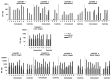
When introduced in the 1990s, immunization with DNA plasmids was considered potentially revolutionary for vaccine development, particularly for vaccines intended to induce protective CD8 T cell responses against multiple antigens. We conducted, in 1997-1998, the first clinical trial in healthy humans of a DNA vaccine, a single plasmid encoding Plasmodium falciparum circumsporozoite protein (PfCSP), as an initial step toward developing a multi-antigen malaria vaccine targeting the liver stages of the parasite. As the next step, we conducted in 2000-2001 a clinical trial of a five-plasmid mixture called MuStDO5 encoding pre-erythrocytic antigens PfCSP, PfSSP2/TRAP, PfEXP1, PfLSA1 and PfLSA3. Thirty-two, malaria-naïve, adult volunteers were enrolled sequentially into four cohorts receiving a mixture of 500 μg of each plasmid plus escalating doses (0, 20, 100 or 500 μg) of a sixth plasmid encoding human granulocyte macrophage-colony stimulating factor (hGM-CSF). Three doses of each formulation were administered intramuscularly by needle-less jet injection at 0, 4 and 8 weeks, and each cohort had controlled human malaria infection administered by five mosquito bites 18 d later. The vaccine was safe and well-tolerated, inducing moderate antigen-specific, MHC-restricted T cell interferon-γ responses but no antibodies. Although no volunteers were protected, T cell responses were boosted post malaria challenge. This trial demonstrated the MuStDO5 DNA and hGM-CSF plasmids to be safe and modestly immunogenic for T cell responses. It also laid the foundation for priming with DNA plasmids and boosting with recombinant viruses, an approach known for nearly 15 y to enhance the immunogenicity and protective efficacy of DNA vaccines.
Keywords: DNA vaccine; GM-CSF; Plasmodium falciparum; clinical trials; controlled human malaria infection; malaria; malaria challenge; malaria vaccine; vaccine safety.
Figures





Similar articles
-
A three-antigen Plasmodium falciparum DNA prime-Adenovirus boost malaria vaccine regimen is superior to a two-antigen regimen and protects against controlled human malaria infection in healthy malaria-naïve adults.PLoS One. 2021 Sep 8;16(9):e0256980. doi: 10.1371/journal.pone.0256980. eCollection 2021. PLoS One. 2021. PMID: 34495988 Free PMC article. Clinical Trial.
-
Boosting of DNA vaccine-elicited gamma interferon responses in humans by exposure to malaria parasites.Infect Immun. 2005 May;73(5):2863-72. doi: 10.1128/IAI.73.5.2863-2872.2005. Infect Immun. 2005. PMID: 15845492 Free PMC article. Clinical Trial.
-
Liver-Directed AAV8 Booster Vaccine Expressing Plasmodium falciparum Antigen Following Adenovirus Vaccine Priming Elicits Sterile Protection in a Murine Model.Front Immunol. 2021 Jun 23;12:612910. doi: 10.3389/fimmu.2021.612910. eCollection 2021. Front Immunol. 2021. PMID: 34248928 Free PMC article.
-
Current Challenges in the Identification of Pre-Erythrocytic Malaria Vaccine Candidate Antigens.Front Immunol. 2020 Feb 21;11:190. doi: 10.3389/fimmu.2020.00190. eCollection 2020. Front Immunol. 2020. PMID: 32153565 Free PMC article. Review.
-
Toward clinical trials of DNA vaccines against malaria.Immunol Cell Biol. 1997 Aug;75(4):376-81. doi: 10.1038/icb.1997.59. Immunol Cell Biol. 1997. PMID: 9315481 Review.
Cited by
-
Direct venous inoculation of Plasmodium falciparum sporozoites for controlled human malaria infection: a dose-finding trial in two centres.Malar J. 2015 Mar 18;14:117. doi: 10.1186/s12936-015-0628-0. Malar J. 2015. PMID: 25889522 Free PMC article. Clinical Trial.
-
External quality assurance of malaria nucleic acid testing for clinical trials and eradication surveillance.PLoS One. 2014 May 16;9(5):e97398. doi: 10.1371/journal.pone.0097398. eCollection 2014. PLoS One. 2014. PMID: 24838112 Free PMC article.
-
The march toward malaria vaccines.Vaccine. 2015 Nov 27;33 Suppl 4(Suppl 4):D13-23. doi: 10.1016/j.vaccine.2015.07.091. Epub 2015 Aug 29. Vaccine. 2015. PMID: 26324116 Free PMC article. Review.
-
A three-antigen Plasmodium falciparum DNA prime-Adenovirus boost malaria vaccine regimen is superior to a two-antigen regimen and protects against controlled human malaria infection in healthy malaria-naïve adults.PLoS One. 2021 Sep 8;16(9):e0256980. doi: 10.1371/journal.pone.0256980. eCollection 2021. PLoS One. 2021. PMID: 34495988 Free PMC article. Clinical Trial.
-
Pandemic Preparedness Against Influenza: DNA Vaccine for Rapid Relief.Front Immunol. 2021 Oct 8;12:747032. doi: 10.3389/fimmu.2021.747032. eCollection 2021. Front Immunol. 2021. PMID: 34691056 Free PMC article.
References
-
- World Malaria Report WHO. 2011
-
- Clyde DF. Immunization of man against falciparum and vivax malaria by use of attenuated sporozoites. Am J Trop Med Hyg. 1975;24:397–401. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
