Frequency modulation entrains slow neural oscillations and optimizes human listening behavior
- PMID: 23151506
- PMCID: PMC3523826
- DOI: 10.1073/pnas.1213390109
Frequency modulation entrains slow neural oscillations and optimizes human listening behavior
Abstract
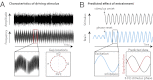
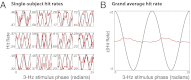
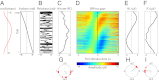
The human ability to continuously track dynamic environmental stimuli, in particular speech, is proposed to profit from "entrainment" of endogenous neural oscillations, which involves phase reorganization such that "optimal" phase comes into line with temporally expected critical events, resulting in improved processing. The current experiment goes beyond previous work in this domain by addressing two thus far unanswered questions. First, how general is neural entrainment to environmental rhythms: Can neural oscillations be entrained by temporal dynamics of ongoing rhythmic stimuli without abrupt onsets? Second, does neural entrainment optimize performance of the perceptual system: Does human auditory perception benefit from neural phase reorganization? In a human electroencephalography study, listeners detected short gaps distributed uniformly with respect to the phase angle of a 3-Hz frequency-modulated stimulus. Listeners' ability to detect gaps in the frequency-modulated sound was not uniformly distributed in time, but clustered in certain preferred phases of the modulation. Moreover, the optimal stimulus phase was individually determined by the neural delta oscillation entrained by the stimulus. Finally, delta phase predicted behavior better than stimulus phase or the event-related potential after the gap. This study demonstrates behavioral benefits of phase realignment in response to frequency-modulated auditory stimuli, overall suggesting that frequency fluctuations in natural environmental input provide a pacing signal for endogenous neural oscillations, thereby influencing perceptual processing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Bishop GH. Cyclic changes in the excitability of the optic pathway of the rabbit. Am J Physiol. 1933;103:213–224.
-
- Buzsáki G, Draguhn A. Neuronal oscillations in cortical networks. Science. 2004;304(5679):1926–1929. - PubMed
-
- Lakatos P, et al. An oscillatory hierarchy controlling neuronal excitability and stimulus processing in the auditory cortex. J Neurophysiol. 2005;94(3):1904–1911. - PubMed
-
- Lakatos P, Karmos G, Mehta AD, Ulbert I, Schroeder CE. Entrainment of neuronal oscillations as a mechanism of attentional selection. Science. 2008;320(5872):110–113. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

