Cholesterol increases kinetic, energetic, and mechanical stability of the human β2-adrenergic receptor
- PMID: 23151510
- PMCID: PMC3528585
- DOI: 10.1073/pnas.1210373109
Cholesterol increases kinetic, energetic, and mechanical stability of the human β2-adrenergic receptor
Abstract
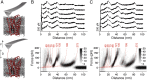
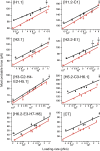
The steroid cholesterol is an essential component of eukaryotic membranes, and it functionally modulates membrane proteins, including G protein-coupled receptors. To reveal insight into how cholesterol modulates G protein-coupled receptors, we have used dynamic single-molecule force spectroscopy to quantify the mechanical strength and flexibility, conformational variability, and kinetic and energetic stability of structural segments stabilizing the human β(2)-adrenergic receptor (β(2)AR) in the absence and presence of the cholesterol analog cholesteryl hemisuccinate (CHS). CHS considerably increased the kinetic, energetic, and mechanical stability of almost every structural segment at sufficient magnitude to alter the structure and functional relationship of β(2)AR. One exception was the structural core segment of β(2)AR, which establishes multiple ligand binding sites, and its properties were not significantly influenced by CHS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
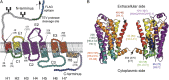


References
-
- Milligan G, Svoboda P, Brown CM. Why are there so many adrenoceptor subtypes? Biochem Pharmacol. 1994;48(6):1059–1071. - PubMed
-
- Takeda S, Kadowaki S, Haga T, Takaesu H, Mitaku S. Identification of G protein-coupled receptor genes from the human genome sequence. FEBS Lett. 2002;520(1-3):97–101. - PubMed
-
- Bai TR. Beta 2 adrenergic receptors in asthma: A current perspective. Lung. 1992;170(3):125–141. - PubMed
-
- Barnes PJ. Beta-adrenoceptors on smooth muscle, nerves and inflammatory cells. Life Sci. 1993;52(26):2101–2109. - PubMed
-
- Smiley RM, Finster M. Do receptors get pregnant too? Adrenergic receptor alterations in human pregnancy. J Matern Fetal Med. 1996;5(3):106–114. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

