Potent activity of the Hsp90 inhibitor ganetespib in prostate cancer cells irrespective of androgen receptor status or variant receptor expression
- PMID: 23152004
- PMCID: PMC3583620
- DOI: 10.3892/ijo.2012.1698
Potent activity of the Hsp90 inhibitor ganetespib in prostate cancer cells irrespective of androgen receptor status or variant receptor expression
Abstract
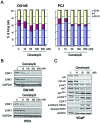
Androgen ablation therapy represents the first line of therapeutic intervention in men with advanced or recurrent prostate tumors. However, the incomplete efficacy and lack of durable response to this clinical strategy highlights an urgent need for alternative treatment options to improve patient outcomes. Targeting the molecular chaperone heat shock protein 90 (Hsp90) represents a potential avenue for therapeutic intervention as its inhibition results in the coordinate blockade of multiple oncogenic signaling pathways in cancer cells. Moreover, Hsp90 is essential for the stability and function of numerous client proteins, a number of which have been causally implicated in the pathogenesis of prostate cancer, including the androgen receptor (AR). Here, we examined the preclinical activity of ganetespib, a small molecule inhibitor of Hsp90, in a panel of prostate cancer cell lines. Ganetespib potently decreased viability in all lines, irrespective of their androgen sensitivity or receptor status, and more effectively than the ansamycin inhibitor 17-allylamino-17-demethoxygeldanamycin (17-AAG). Interestingly, while ganetespib exposure decreased AR expression and activation, the constitutively active V7 truncated isoform of the receptor was unaffected by Hsp90 inhibition. Mechanistically, ganetespib exerted concomitant effects on mitogenic and survival pathways, as well as direct modulation of cell cycle regulators, to induce growth arrest and apoptosis. Further, ganetespib displayed robust antitumor efficacy in both AR-negative and positive xenografts, including those derived from the 22Rv1 prostate cancer cell line that co-expresses full-length and variant receptors. Together these data suggest that further investigation of ganetespib as a new therapeutic treatment for prostate cancer patients is warranted.
Figures




Similar articles
-
Ganetespib, a unique triazolone-containing Hsp90 inhibitor, exhibits potent antitumor activity and a superior safety profile for cancer therapy.Mol Cancer Ther. 2012 Feb;11(2):475-84. doi: 10.1158/1535-7163.MCT-11-0755. Epub 2011 Dec 5. Mol Cancer Ther. 2012. PMID: 22144665
-
Co-targeting AR and HSP90 suppresses prostate cancer cell growth and prevents resistance mechanisms.Endocr Relat Cancer. 2015 Oct;22(5):805-18. doi: 10.1530/ERC-14-0541. Epub 2015 Jul 17. Endocr Relat Cancer. 2015. PMID: 26187127
-
Ganetespib (STA-9090), a nongeldanamycin HSP90 inhibitor, has potent antitumor activity in in vitro and in vivo models of non-small cell lung cancer.Clin Cancer Res. 2012 Sep 15;18(18):4973-85. doi: 10.1158/1078-0432.CCR-11-2967. Epub 2012 Jul 17. Clin Cancer Res. 2012. PMID: 22806877 Free PMC article.
-
Hsp90 as a therapeutic target in prostate cancer.Semin Oncol. 2003 Oct;30(5):709-16. doi: 10.1016/s0093-7754(03)00346-4. Semin Oncol. 2003. PMID: 14571418 Review.
-
Role of Ganetespib, an HSP90 Inhibitor, in Cancer Therapy: From Molecular Mechanisms to Clinical Practice.Int J Mol Sci. 2023 Mar 6;24(5):5014. doi: 10.3390/ijms24055014. Int J Mol Sci. 2023. PMID: 36902446 Free PMC article. Review.
Cited by
-
The HSP90 inhibitor ganetespib has chemosensitizer and radiosensitizer activity in colorectal cancer.Invest New Drugs. 2014 Aug;32(4):577-86. doi: 10.1007/s10637-014-0095-4. Epub 2014 Apr 1. Invest New Drugs. 2014. PMID: 24682747 Free PMC article.
-
Senolytic compounds control a distinct fate of androgen receptor agonist- and antagonist-induced cellular senescent LNCaP prostate cancer cells.Cell Biosci. 2020 Apr 25;10:59. doi: 10.1186/s13578-020-00422-2. eCollection 2020. Cell Biosci. 2020. PMID: 32351687 Free PMC article.
-
Moving Beyond the Androgen Receptor (AR): Targeting AR-Interacting Proteins to Treat Prostate Cancer.Horm Cancer. 2016 Apr;7(2):84-103. doi: 10.1007/s12672-015-0239-9. Epub 2016 Jan 4. Horm Cancer. 2016. PMID: 26728473 Free PMC article. Review.
-
The evolution and polymorphism of mono-amino acid repeats in androgen receptor and their regulatory role in health and disease.Front Med (Lausanne). 2022 Oct 20;9:1019803. doi: 10.3389/fmed.2022.1019803. eCollection 2022. Front Med (Lausanne). 2022. PMID: 36388907 Free PMC article. Review.
-
Constitutively-active androgen receptor variants function independently of the HSP90 chaperone but do not confer resistance to HSP90 inhibitors.Oncotarget. 2013 May;4(5):691-704. doi: 10.18632/oncotarget.975. Oncotarget. 2013. PMID: 23674566 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

