ZNF143 transcription factor mediates cell survival through upregulation of the GPX1 activity in the mitochondrial respiratory dysfunction
- PMID: 23152058
- PMCID: PMC3542592
- DOI: 10.1038/cddis.2012.156
ZNF143 transcription factor mediates cell survival through upregulation of the GPX1 activity in the mitochondrial respiratory dysfunction
Abstract
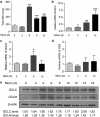
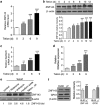
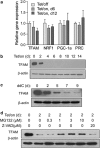
Mitochondrial respiratory dysfunction has intimate relationship with redox regulation. The key mechanism about how the mitochondrial respiration-defective cells survive oxidative stress is still elusive. Here, we report that transcription factor zinc-finger protein 143 (ZNF143) expression and glutathione peroxidase (GPX) activity are markedly increased in the mitochondrial respiratory-defective cells induced by dominant-negative DNA polymerase γ (POLGdn). In this work, investigation of the cellular antioxidant glutathione (GSH) and enzyme GPX activity in the mitochondrial dysfunction revealed the presence of an increased synthesis of GSH through the activation of GCLC (glutamate-cysteine ligase catalytic subunit) and GCLM (glutamate-cysteine ligase regulatory subunit) gene expression, and also a positive upregulation of glutathione peroxidase 1 (GPX1) activity by the transcription factor ZNF143. Significant increase in gene expression of SepSecS, the key enzyme responsible for selenocysteine transfer RNA (tRNA) synthesis, further confirmed the activation of the selenocysteine synthesis pathway. By using both GPX1 and ZNF143 knockdown, we provided insight into the involvement of ZNF143 in promoting GPX1 activity and protecting cells from oxidative damage and cisplatin treatment in the mitochondrial dysfunction. Furthermore, we reported the possible regulation of mitochondrial transcription factor A (TFAM) in the mitochondrial dysfunction. Our findings delineate an important antioxidant survival pathway that allows the mitochondrial-defective cells to survive oxidative stress and cisplatin treatment.
Figures





Similar articles
-
Succinobucol, a Lipid-Lowering Drug, Protects Against 3-Nitropropionic Acid-Induced Mitochondrial Dysfunction and Oxidative Stress in SH-SY5Y Cells via Upregulation of Glutathione Levels and Glutamate Cysteine Ligase Activity.Mol Neurobiol. 2016 Mar;53(2):1280-1295. doi: 10.1007/s12035-014-9086-x. Epub 2015 Jan 27. Mol Neurobiol. 2016. PMID: 25619973
-
Mitochondrial GPx1 decreases induced but not basal oxidative damage to mtDNA in T47D cells.Biochem Biophys Res Commun. 2000 Jun 7;272(2):416-22. doi: 10.1006/bbrc.2000.2800. Biochem Biophys Res Commun. 2000. PMID: 10833429
-
Design, Synthesis, and In Vitro Evaluation of a Novel Probucol Derivative: Protective Activity in Neuronal Cells Through GPx Upregulation.Mol Neurobiol. 2018 Oct;55(10):7619-7634. doi: 10.1007/s12035-018-0939-6. Epub 2018 Feb 12. Mol Neurobiol. 2018. PMID: 29430618
-
The role of glutathione peroxidase-1 in health and disease.Free Radic Biol Med. 2022 Aug 1;188:146-161. doi: 10.1016/j.freeradbiomed.2022.06.004. Epub 2022 Jun 9. Free Radic Biol Med. 2022. PMID: 35691509 Free PMC article. Review.
-
Glutathione peroxidase-1 in health and disease: from molecular mechanisms to therapeutic opportunities.Antioxid Redox Signal. 2011 Oct 1;15(7):1957-97. doi: 10.1089/ars.2010.3586. Epub 2011 Apr 10. Antioxid Redox Signal. 2011. PMID: 21087145 Free PMC article. Review.
Cited by
-
Estrogen signaling as a bridge between the nucleus and mitochondria in cardiovascular diseases.Front Cell Dev Biol. 2022 Sep 14;10:968373. doi: 10.3389/fcell.2022.968373. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36187489 Free PMC article. Review.
-
Role of glutathione peroxidase 1 in glucose and lipid metabolism-related diseases.Free Radic Biol Med. 2018 Nov 1;127:108-115. doi: 10.1016/j.freeradbiomed.2018.05.077. Epub 2018 May 22. Free Radic Biol Med. 2018. PMID: 29800654 Free PMC article. Review.
-
Effects of alternative splicing events and transcriptome changes on kidney stone formation.Urolithiasis. 2022 Apr;50(2):131-140. doi: 10.1007/s00240-021-01293-z. Epub 2022 Jan 8. Urolithiasis. 2022. PMID: 34997271 Free PMC article.
-
Determining the genetic basis of anthracycline-cardiotoxicity by molecular response QTL mapping in induced cardiomyocytes.Elife. 2018 May 8;7:e33480. doi: 10.7554/eLife.33480. Elife. 2018. PMID: 29737278 Free PMC article.
-
ZNF143 is a transcriptional regulator of nuclear-encoded mitochondrial genes that acts independently of looping and CTCF.Mol Cell. 2025 Jan 2;85(1):24-41.e11. doi: 10.1016/j.molcel.2024.11.031. Epub 2024 Dec 20. Mol Cell. 2025. PMID: 39708805 Free PMC article.
References
-
- Shen Q, Chu FF, Newburger PE. Sequences in the 3′-untranslated region of the human cellular glutathione peroxidase gene are necessary and sufficient for selenocysteine incorporation at the UGA codon. J Biol Chem. 1993;268:11463–11469. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

