Δ9-tetrahydrocannabinol impairs the inflammatory response to influenza infection: role of antigen-presenting cells and the cannabinoid receptors 1 and 2
- PMID: 23152191
- PMCID: PMC3551428
- DOI: 10.1093/toxsci/kfs315
Δ9-tetrahydrocannabinol impairs the inflammatory response to influenza infection: role of antigen-presenting cells and the cannabinoid receptors 1 and 2
Abstract
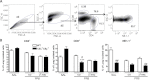
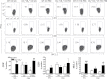
Δ(9)-tetrahydrocannabinol (Δ(9)-THC) has potent immune modulatory properties and can impair pathogen-induced immune defenses, which in part have been attributed to ligation of the cannabinoid receptors 1 (CB(1)) and 2 (CB(2)). Most recently, dendritic cells (DC) were identified for their potential to enhance influenza-induced immunopathology in mice lacking CB(1) and CB(2) (CB(1) (-/-)CB(2) (-/-)). This study focused on the modulation of the inflammatory immune response to influenza by Δ(9)-THC and the role of CB(1) and/or CB(2) as receptor targets for Δ(9)-THC. C57Bl/6 (wild type) and CB(1) (-/-)CB(2) (-/-) mice were administered Δ(9)-THC (75 mg/kg) surrounding the intranasal instillation of A/PR/8/34 influenza virus. Three days post infection (dpi), Δ(9)-THC broadly decreased expression levels of mRNA induced by the innate immune response to influenza, suppressed the percentage of interferon-gamma (IFN-γ)-producing CD4(+) and interleukin-17-producing NK1.1(+) cells, and reduced the influx of antigen-presenting cells (APC), including inflammatory myeloid cells and monocytes/macrophages, into the lung in a CB(1)- and/or CB(2)-dependent manner. Δ(9)-THC had little effect on the expression of CD86, major histocompatibility complex I (MHC I), and MHC II by APC isolated from the lung. In vitro studies demonstrated that lipopolysaccharide (LPS)-induced maturation was suppressed by Δ(9)-THC in bone marrow-derived DC (bmDC). Furthermore, antigen-specific IFN-γ production by CD8(+) T cells after coculture was reduced by Δ(9)-THC treatment of bmDC in a CB(1)- and/or CB(2)-dependent manner. Collectively, these studies suggest that Δ(9)-THC potently suppresses myeloid cell immune function, in a manner involving CB(1) and/or CB(2), thereby impairing immune responses to influenza infection.
Figures







Similar articles
-
Targeted deletion of cannabinoid receptors CB1 and CB2 produced enhanced inflammatory responses to influenza A/PR/8/34 in the absence and presence of Delta9-tetrahydrocannabinol.J Leukoc Biol. 2008 Mar;83(3):785-96. doi: 10.1189/jlb.0907618. Epub 2007 Dec 11. J Leukoc Biol. 2008. PMID: 18073275
-
Δ9-tetrahydrocannabinol suppresses cytotoxic T lymphocyte function independent of CB1 and CB 2, disrupting early activation events.J Neuroimmune Pharmacol. 2012 Dec;7(4):843-55. doi: 10.1007/s11481-011-9293-4. Epub 2011 Jul 26. J Neuroimmune Pharmacol. 2012. PMID: 21789506 Free PMC article.
-
Role of cannabinoid receptors in Delta-9-tetrahydrocannabinol suppression of IL-12p40 in mouse bone marrow-derived dendritic cells infected with Legionella pneumophila.Eur J Pharmacol. 2006 Feb 17;532(1-2):170-7. doi: 10.1016/j.ejphar.2005.12.040. Epub 2006 Jan 27. Eur J Pharmacol. 2006. PMID: 16443217
-
Update on the role of cannabinoid receptors after ischemic stroke.Mediators Inflamm. 2012;2012:824093. doi: 10.1155/2012/824093. Epub 2012 Apr 5. Mediators Inflamm. 2012. PMID: 22577257 Free PMC article. Review.
-
An Agathokakological Tale of Δ9-THC: Exploration of Possible Biological Targets.Curr Drug Targets. 2021;22(7):823-834. doi: 10.2174/1389450121666201001123515. Curr Drug Targets. 2021. PMID: 33001012 Review.
Cited by
-
Interaction between Cannabinoid System and Toll-Like Receptors Controls Inflammation.Mediators Inflamm. 2016;2016:5831315. doi: 10.1155/2016/5831315. Epub 2016 Aug 11. Mediators Inflamm. 2016. PMID: 27597805 Free PMC article. Review.
-
Impact of Cannabis, Cannabinoids, and Endocannabinoids in the Lungs.Front Pharmacol. 2016 Sep 15;7:317. doi: 10.3389/fphar.2016.00317. eCollection 2016. Front Pharmacol. 2016. PMID: 27695418 Free PMC article. Review.
-
Δ9-Tetrahydrocannabinol Suppresses Monocyte-Mediated Astrocyte Production of Monocyte Chemoattractant Protein 1 and Interleukin-6 in a Toll-Like Receptor 7-Stimulated Human Coculture.J Pharmacol Exp Ther. 2019 Oct;371(1):191-201. doi: 10.1124/jpet.119.260661. Epub 2019 Aug 5. J Pharmacol Exp Ther. 2019. PMID: 31383729 Free PMC article.
-
Considerations for Cannabinoids in Perioperative Care by Anesthesiologists.J Clin Med. 2022 Jan 22;11(3):558. doi: 10.3390/jcm11030558. J Clin Med. 2022. PMID: 35160010 Free PMC article. Review.
-
Weed, sex and influenza.ERJ Open Res. 2023 Nov 27;9(6):00619-2023. doi: 10.1183/23120541.00619-2023. eCollection 2023 Nov. ERJ Open Res. 2023. PMID: 38020565 Free PMC article.
References
-
- Allen S. J., Crown S. E., Handel T. M. (2007). Chemokine: Receptor structure, interactions, and antagonism. Annu. Rev. Immunol. 25, 787–820 - PubMed
-
- Asselin-Paturel C., Boonstra A., Dalod M., Durand I., Yessaad N., Dezutter-Dambuyant C., Vicari A., O’Garra A., Biron C., Brière F, et al. (2001). Mouse type I IFN-producing cells are immature APCs with plasmacytoid morphology. Nat. Immunol. 2, 1144–1150 - PubMed
-
- Auffray C., Sieweke M. H., Geissmann F. (2009). Blood monocytes: Development, heterogeneity, and relationship with dendritic cells. Annu. Rev. Immunol. 27, 669–692 - PubMed
-
- Azorlosa J. L., Heishman S. J., Stitzer M. L., Mahaffey J. M. (1992). Marijuana smoking: Effect of varying delta 9-tetrahydrocannabinol content and number of puffs. J. Pharmacol. Exp. Ther. 261, 114–122 - PubMed
-
- Banchereau J., Steinman R. M. (1998). Dendritic cells and the control of immunity. Nature 392, 245–252 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

