Early versus delayed selective surfactant treatment for neonatal respiratory distress syndrome
- PMID: 23152207
- PMCID: PMC7057030
- DOI: 10.1002/14651858.CD001456.pub2
Early versus delayed selective surfactant treatment for neonatal respiratory distress syndrome
Abstract
Background: Clinical trials have confirmed that surfactant therapy is effective in improving the immediate need for respiratory support and the clinical outcome of premature newborns. Trials have studied a wide variety of surfactant preparations used either to prevent (prophylactic or delivery room administration) or treat (selective or rescue administration) respiratory distress syndrome (RDS). Using either treatment strategy, significant reductions in the incidence of pneumothorax, as well as significant improvement in survival, have been noted. It is unclear whether there are any advantages to treating infants with respiratory insufficiency earlier in the course of RDS.
Objectives: To compare the effects of early versus delayed selective surfactant therapy for newborns intubated for respiratory distress within the first two hours of life. Planned subgroup analyses included separate comparisons for studies utilizing natural surfactant extract and synthetic surfactant.
Search methods: We searched the Oxford Database of Perinatal Trials, MEDLINE (MeSH terms: pulmonary surfactant; text word: early; limits: age, newborn: publication type, clinical trial), PubMed, abstracts, conference and symposia proceedings, expert informants, and journal handsearching in the English language. For the updated search in April 2012 we searched the Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library, 2012, Issue 1) and PubMed (January 1997 to April 2012).
Selection criteria: Randomized and quasi-randomized controlled clinical trials comparing early selective surfactant administration (surfactant administration via the endotracheal tube in infants intubated for respiratory distress, not specifically for surfactant dosage) within the first two hours of life versus delayed selective surfactant administration to infants with established RDS were considered for review.
Data collection and analysis: Data regarding clinical outcomes were excerpted from the reports of the clinical trials by the review authors. Subgroup analyses were performed based on type of surfactant preparation, gestational age, and exposure to prenatal steroids. Data analysis was performed in accordance with the standards of the Cochrane Neonatal Review Group.
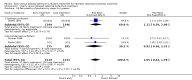
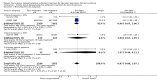
Main results: Six randomized controlled trials met selection criteria. Two of the trials utilized synthetic surfactant (Exosurf Neonatal) and four utilized animal-derived surfactant preparations.The meta-analyses demonstrate significant reductions in the risk of neonatal mortality (typical risk ratio (RR) 0.84; 95% confidence interval (CI) 0.74 to 0.95; typical risk difference (RD) -0.04; 95% CI -0.06 to -0.01; 6 studies; 3577 infants), chronic lung disease (typical RR 0.69; 95% CI 0.55 to 0.86; typical RD -0.04; 95% CI -0.06 to -0.01; 3 studies; 3041 infants), and chronic lung disease or death at 36 weeks (typical RR 0.83; 95% CI 0.75 to 0.91; typical RD -0.06; 95% CI -0.09 to -0.03; 3 studies; 3050 infants) associated with early treatment of intubated infants with RDS.Intubated infants randomized to early selective surfactant administration also demonstrated a decreased risk of acute lung injury including a decreased risk of pneumothorax (typical RR 0.69; 95% CI 0.59 to 0.82; typical RD -0.05; 95% CI -0.08 to -0.03; 5 studies; 3545 infants), pulmonary interstitial emphysema (typical RR 0.60; 95% CI 0.41 to 0.89; typical RD -0.06; 95% CI -0.10 to -0.02; 3 studies; 780 infants), and overall air leak syndromes (typical RR 0.61; 95% CI 0.48 to 0.78; typical RD -0.18; 95% CI -0.26 to -0.09; 2 studies; 463 infants).A trend toward risk reduction for bronchopulmonary dysplasia (BPD) or death at 28 days was also evident (typical RR 0.94; 95% CI 0.88 to 1.00; typical RD -0.04; 95% CI -0.07 to -0.00; 3 studies; 3039 infants). No differences in other complications of RDS or prematurity were noted.Only two studies reported on infants under 30 weeks' gestation. Decreased risk of neonatal mortality and chronic lung disease or death at 36 weeks' postmenstrual age was noted.
Authors' conclusions: Early selective surfactant administration given to infants with RDS requiring assisted ventilation leads to a decreased risk of acute pulmonary injury (decreased risk of pneumothorax and pulmonary interstitial emphysema) and a decreased risk of neonatal mortality and chronic lung disease compared to delaying treatment of such infants until they develop worsening RDS.
Conflict of interest statement
Dr. R. Soll has previously acted as a paid consultant and invited speaker for several of the pharmaceutical companies that manufacture surfactant preparations (Abbott Laboratories, Ross Laboratories, Chiesi Pharmaceuticals, Dey Laboratories, Burroughs Wellcome).
Felicia L Bahadue has no conflicts of interest to declare.
Figures



























Update of
-
Early versus delayed selective surfactant treatment for neonatal respiratory distress syndrome.Cochrane Database Syst Rev. 2000;(2):CD001456. doi: 10.1002/14651858.CD001456. Cochrane Database Syst Rev. 2000. Update in: Cochrane Database Syst Rev. 2012 Nov 14;11:CD001456. doi: 10.1002/14651858.CD001456.pub2. PMID: 10796266 Updated.
References
References to studies included in this review
European Study 1992 {published data only}
-
- European Exosurf Study Group. Early or selective surfactant (Colfosceril Palmitate, Exosurf) for intubated babies at 26 to 29 weeks gestation: a European double‐blind trial with sequential analysis. Online Journal of Current Clinical Trials 1992:Doc. No. 28. - PubMed
Gortner 1998 {published data only}
-
- Gortner L, Wauer RR, Hammer H, Stock GJ, Heitmann F, Reiter HL, et al. Early versus late surfactant treatment in preterm infants of 27 to 32 weeks' gestational age: a multicenter controlled clinical trial. Pediatrics 1998;102(5):1153‐60. - PubMed
-
- Hentschel R, Dittrich F, Hilgendorff A, Wauer R, Westmeier M, Gortner L. Neurodevelopmental outcome and pulmonary morbidity two years after early versus late surfactant treatment: does it really differ?. Acta Paediatroca 2009;98(4):654‐9. - PubMed
Konishi 1992 {published data only}
-
- Konishi M, Fujiwara T, Chida S, Maeta H, Shimada S, Kasai T, et al. A prospective randomized trial of early versus late administration of a single dose of surfactant‐TA. Early Human Development 1992;29(1‐3):275‐82. - PubMed
Lefort 2003 {published and unpublished data}
-
- Lefort S, Diniz EM, Vaz FA. Clinical course of premature infants intubated in the delivery room, submitted or not to porcine‐derived lung surfactant therapy within the first hour of life. Journal of Maternal‐Fetal & Neonatal Medicine 2003;14(3):187‐96. - PubMed
OSIRIS 1992 {published data only}
-
- The OSIRIS Collaborative Group. Early versus delayed neonatal administration of a synthetic surfactant ‐ the judgement of OSIRIS. The OSIRIS Collaborative Group (open study of infants at high risk of or with respiratory insufficiency ‐ the role of surfactant. Lancet 1992;340(8832):1363‐9. - PubMed
Plavka 2002 {published and unpublished data}
-
- Plavka R, Kopecký P, Sebron V, Leiská A, Svihovec P, Ruffer J, et al. Early versus delayed surfactant administration in extremely premature neonates with respiratory distress syndrome ventilated by high‐frequency oscillatory ventilation. Intensive Care Medicine 2002;28(10):1483‐90. - PubMed
References to studies excluded from this review
Bevilacqua 1996 {published data only}
-
- Bevilacqua G, Parmigiani S, Robertson B. Prophylaxis of respiratory distress syndrome by treatment with modified porcine surfactant at birth: a multicentre prospective randomized trial. Journal of Perinatal Medicine 1996;24(6):609‐20. - PubMed
Bevilacqua 1997 {published data only}
-
- Bevilacqua G, Chernev T, Parmigiani S, Iarakova N, Gaioni L, Volante E, et al. Use of surfactant for prophylaxis versus rescue treatment of respiratory distress syndrome: experience from an Italian‐Bulgarian trial. Acta Bio‐Medica de L'Ateneo parmense 1997;68(Suppl 1):47‐54. - PubMed
Dunn 1991 {published data only}
-
- Dunn MS, Shennan AT, Zayack D, Possmayer F. Bovine surfactant replacement therapy in neonates of less than 30 weeks' gestation: a randomized controlled trial of prophylaxis vs treatment. Pediatrics 1991;87(3):377‐86. - PubMed
Dunn 2011 {published data only}
-
- Dunn MS, Kaempf J, Klerk A, Klerk R, Reilly M, Howard D, et al. Randomized trial comparing 3 approaches to the initial respiratory management of preterm neonates. Pediatrics 2011;128(5):e1069‐76. - PubMed
Egberts 1993 {published data only}
-
- Egberts J, Winter JP, Sedin G, Kleine MJ, Broberger U, Bel F, et al. Comparison of prophylaxis and rescue treatment with Curosurf in neonates less than 30 weeks gestation: a randomized trial. Pediatrics 1993;92(6):768‐74. - PubMed
Escobedo 2004 {published data only}
-
- Escobedo MB, Gunkel JH, Kennedy KA, Shattuck KE, Sánchez PJ, Seidner S, et al. Early surfactant for neonates with mild to moderate respiratory distress syndrome: a multicenter, randomized trial. Journal of Pediatrics 2004;144(6):804‐8. - PubMed
Figueras‐Aloy 2001 {published data only}
-
- Figueras‐Aloy J, Quero J, Carbonell‐Estrany X, Ginovart G, Pérez‐Rodríguez J, Raspall F, et al. Early administration of the second dose of surfactant (beractant) in the treatment of severe hyaline membrane disease. Acta Pediatrica 2001;90(3):296‐301. - PubMed
Göpel 2011 {published data only}
-
- Göpel W, Kribs A, Ziegler A, Laux R, Hoehn T, Wieg C, et al. Avoidance of mechanical ventilation by surfactant treatment of spontaneously breathing preterm infants (AMV): an open‐label, randomised, controlled trial. Lancet 2011;378(9803):1627‐34. - PubMed
Iarŭkova 1999 {published data only}
-
- Iarŭkova N, Vakrilova L, Slŭncheva B, Dancheva S, Popivanova A, Emilova Z, et al. Administration of exogenous surfactant in very low birth weight infants with RDS. Akuserstvo i Ginekologija 1999;38(1):23‐6. - PubMed
Kattwinkel 1993 {published data only}
-
- Kattwinkel J, Bloom BT, Delmore P, Davis CL, Farrell E, Friss H, et al. Prophylactic administration of calf lung surfactant extract is more effective than early treatment of respiratory distress syndrome in neonates of 29 through 32 weeks' gestation. Pediatrics 1993;92(1):90‐8. - PubMed
Kattwinkel 2000 {published data only}
-
- Kattwinkel J, Bloom BT, Delmore P, Glick C, Brown D, Lopez S, et al. High‐versus low‐threshold surfactant retreatment for neonatal respiratory distress syndrome. Pediatrics 2000;106(2 Pt 1):282‐8. - PubMed
Kendig 1991 {published data only}
-
- Kendig JW, Notter RH, Cox C, Reubens LJ, Davis JM, Maniscalco WM, et al. A comparison of surfactant as immediate prophylaxis and as rescue therapy in newborns of less than 30 weeks' gestation. New England Journal of Medicine 1991;324(13):865‐71. - PubMed
Köksal 2009 {published data only}
-
- Köksal N, Akpinar R, Cetinkaya M. Early administration of the second surfactant dose in preterm infants with severe respiratory distress syndrome. Turkis Journal of Pediatrics 2009;51(6):556‐64. - PubMed
Merritt 1991 {published data only}
-
- Merritt TA, Hallman M, Berry C, Pohjavuori M, Edwards DK, Jaaskelainen J, et al. Randomized, placebo‐controlled trial of human surfactant given at birth vs rescue administration in very low birthweight infants with lung immaturity. Journal of Pediatrics 1991;118(4 Pt 1):581‐94. - PubMed
Morley 2008 {published data only}
-
- Morley CJ, Davis PG, Doyle LW, Brion LP, Hascoet JM, Carlin JB, COIN Trial Investigators. Nasal CPAP or intubation at birth for very preterm infants. New England Journal of Medicine 2008;358(7):700‐8. - PubMed
Osborn 2000 {published data only}
-
- Osborn DA, Jeffery HE, Bredemeyer SL, Polverino JM, Reid S. Targeted early rescue surfactant in ventilated preterm infants using the click test. Pediatrics 2000;106(3):E30. - PubMed
Reininger 2005 {published data only}
-
- Reininger A, Khalak R, Kendig JW, Ryan RM, Stevens TP, Reubens L, et al. Surfactant administration by transient intubation in infants 29 to 35 weeks' gestation with respiratory distress syndrome decreases the likelihood of later mechanical ventilation: a randomized controlled trial. Journal of Perinatology 2005;25(11):703‐8. - PubMed
Rojas 2009 {published data only}
-
- Rojas MA, Lozano JM, Rojas MX, Laughon M, Bose CL, Rondon MA, et al. Very early surfactant without mandatory ventilation in premature infants treated with early continuous positive airway pressure: a randomized, controlled trial. Pediatrics 2009;123(1):137‐42. - PubMed
Sandri 2010 {published data only}
-
- Sandri F, Plavka R, Ancora G, Simeoni U, Stranak Z, Martinelli S, et al. Prophylactic or early selective surfactant combined with nCPAP in very preterm infants. Pediatrics 2010;125(6):e1402‐9. - PubMed
SUPPORT 2010 {published data only}
Verder 1999 {published data only}
-
- Verder H, Albertsen P, Ebbesen F, Greisen G, Robertson B, Bertelsen A, et al. Nasal continuous positive airway pressure and early surfactant therapy for respiratory distress syndrome in newborns of less than 30 weeks' gestation. Pediatrics 1999;103(2):E24. - PubMed
Walti 1995 {published data only}
-
- Walti H, Paris‐Llado J, Breart G, Couchard M, and the French Collaborative Multicentre Study Group. Porcine surfactant replacement therapy in newborns of 25‐31 weeks' gestation: a randomized multicentre trial of prophylaxis versus rescue with multiple low doses. Acta Paediatrica 1995;84(8):913‐21. - PubMed
Additional references
Abdel‐Latif 2010
Abdel‐Latif 2011
Abdel‐Latif 2011a
Crowley 2006
Dargaville 2002
El Shahed 2007
Ikegami 1983
-
- Ikegami M, Jacobs H, Jobe A. Surfactant function in respiratory distress syndrome. Journal of Pediatrics 1983;102(3):443‐7. - PubMed
Jobe 1993
-
- Jobe AH, Mitchell BR, Gunkel JH. Beneficial effects of the combined use of prenatal corticosteroids and postnatal surfactant in preterm infants. American Journal of Obstetrics and Gynecology 1993;168(2):508‐13. - PubMed
Nilsson 1978
-
- Nilsson R, Grossman G, Robertson B. Lung surfactant and the pathogenesis of neonatal bronchiolar lesions induced by artificial ventilation. Pediatric Research 1978;12(4 Pt 1):249‐55. - PubMed
Pfister 2007
Pfister 2009
Possmayer 1990
-
- Possmayer F. The role of surfactant‐associated proteins. American Review of Respiratory Disease 1990;142(4):749‐52. - PubMed
RevMan 2011 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Rojas‐Reyes 2012
Schurch 1992
-
- Schürch S, Possmayer F, Cheng S, Cockshutt AM. Pulmonary SP‐A enhances adsorption and appears to induce surface sorting of lipid extract surfactant. American Journal of Physiology 1992;263(2 Pt 1):L210‐8. - PubMed
Seger 2009
Sinclair 1992
-
- Sinclair JC, Bracken M (Editors). Effective Care of the Newborn Infant. Oxford: Oxford University Press, 1992.
Singh 2011
-
- Singh N, Hawley KL, Viswanathan K. Efficacy of porcine versus bovine surfactants for preterm newborns with respiratory distress syndrome: systematic review and meta‐analysis. Pediatrics 2011; Vol. 128, issue 6:e1588‐95. - PubMed
Soll 1992
-
- Soll RF, McQueen MC. Respiratory distress syndrome. In: Sinclair JC, Bracken MB editor(s). Effective Care of the Newborn Infant. Oxford: Oxford University Press, 1992:325‐358.
Soll 1997
Soll 2000
Soll 2001
Soll 2009
Soll 2010
Stevens 2007
-
- Stevens TP, Harrington EW, Blennow M, Soll RF. Early surfactant administration with brief ventilation vs. selective surfactant and continued mechanical ventilation for preterm infants with or at risk for respiratory distress syndrome. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD003063.pub3] - DOI - PMC - PubMed
Tan 2012
Wright 1997
-
- Wright JR. Immunomodulatory functions of surfactant. Physiological Reviews 1997;77(4):931‐62. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

