Recombinant human insulin-like growth factor I (rhIGF-I) for the treatment of amyotrophic lateral sclerosis/motor neuron disease
- PMID: 23152212
- PMCID: PMC11930389
- DOI: 10.1002/14651858.CD002064.pub3
Recombinant human insulin-like growth factor I (rhIGF-I) for the treatment of amyotrophic lateral sclerosis/motor neuron disease
Abstract
Background: Recombinant human insulin-like growth factor I (rhIGF-I) is a possible disease modifying therapy for amyotrophic lateral sclerosis (ALS, which is also known as motor neuron disease (MND)).
Objectives: To examine the efficacy of rhIGF-I in affecting disease progression, impact on measures of functional health status, prolonging survival and delaying the use of surrogates (tracheostomy and mechanical ventilation) to sustain survival in ALS. Occurrence of adverse events was also reviewed.
Search methods: We searched the Cochrane Neuromuscular Disease Group Specialized Register (21 November 2011), CENTRAL (2011, Issue 4), MEDLINE (January 1966 to November 2011) and EMBASE (January 1980 to November 2011) and sought information from the authors of randomised clinical trials and manufacturers of rhIGF-I.
Selection criteria: We considered all randomised controlled clinical trials involving rhIGF-I treatment of adults with definite or probable ALS according to the El Escorial Criteria. The primary outcome measure was change in Appel Amyotrophic Lateral Sclerosis Rating Scale (AALSRS) total score after nine months of treatment and secondary outcome measures were change in AALSRS at 1, 2, 3, 4, 5, 6, 7, 8, 9 months, change in quality of life (Sickness Impact Profile scale), survival and adverse events.
Data collection and analysis: Each author independently graded the risk of bias in the included studies. The lead author extracted data and the other authors checked them. We generated some missing data by making ruler measurements of data in published graphs. We collected data about adverse events from the included trials.
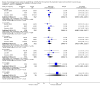
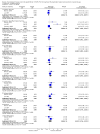
Main results: We identified three randomised controlled trials (RCTs) of rhIGF-I, involving 779 participants, for inclusion in the analysis. In a European trial (183 participants) the mean difference (MD) in change in AALSRS total score after nine months was -3.30 (95% confidence interval (CI) -8.68 to 2.08). In a North American trial (266 participants), the MD after nine months was -6.00 (95% CI -10.99 to -1.01). The combined analysis from both RCTs showed a MD after nine months of -4.75 (95% CI -8.41 to -1.09), a significant difference in favour of the treated group. The secondary outcome measures showed non-significant trends favouring rhIGF-I. There was an increased risk of injection site reactions with rhIGF-I (risk ratio 1.26, 95% CI 1.04 to 1.54). . A second North American trial (330 participants) used a novel primary end point involving manual muscle strength testing. No differences were demonstrated between the treated and placebo groups in this study. All three trials were at high risk of bias.
Authors' conclusions: Meta-analysis revealed a significant difference in favour of rhIGF-I treatment; however, the quality of the evidence from the two included trials was low. A third study showed no difference between treatment and placebo. There is no evidence for increase in survival with IGF1. All three included trials were at high risk of bias.
Conflict of interest statement
Three of the authors (JDM, JHJW and GDB) have accepted speakers' honoraria from several pharmaceutical firms, including Cephalon, Inc, the manufacturer of rhIGF‐I. JHJW was an investigator in the European trial of rhIGF‐I (Borasio 1998) and reviewed a preliminary version of the manuscript but did not participate in data analysis. JDM has experience of the treatment of a small number of ALS patients with rhIGF‐I during the course of an open label safety evaluation funded by Cephalon. GDB had a major role in the European trial of rhIGF‐I and was lead author of the paper in which the results were reported. He received a grant from Cephalon, Inc covering study expenses, as well as travel reimbursement for meetings. He received no support of any kind from Cephalon, Inc. or related companies after the year 2000. MB had no role in any of the trials.
For Dr Mitchell (deceased), declarations of interest are as published in the previous version of this review.
Figures








Update of
-
Recombinant human insulin-like growth factor I (rhIGF-I) for amyotrophic lateral sclerosis/motor neuron disease.Cochrane Database Syst Rev. 2007 Oct 17;(4):CD002064. doi: 10.1002/14651858.CD002064.pub2. Cochrane Database Syst Rev. 2007. Update in: Cochrane Database Syst Rev. 2012 Nov 14;11:CD002064. doi: 10.1002/14651858.CD002064.pub3. PMID: 17943766 Updated.
Similar articles
-
Recombinant human insulin-like growth factor I (rhIGF-I) for amyotrophic lateral sclerosis/motor neuron disease.Cochrane Database Syst Rev. 2007 Oct 17;(4):CD002064. doi: 10.1002/14651858.CD002064.pub2. Cochrane Database Syst Rev. 2007. Update in: Cochrane Database Syst Rev. 2012 Nov 14;11:CD002064. doi: 10.1002/14651858.CD002064.pub3. PMID: 17943766 Updated.
-
Recombinant human insulin-like growth factor I (rhIGF-I) for amyotrophic lateral sclerosis/motor neuron disease.Cochrane Database Syst Rev. 2002;(3):CD002064. doi: 10.1002/14651858.CD002064. Cochrane Database Syst Rev. 2002. Update in: Cochrane Database Syst Rev. 2007 Oct 17;(4):CD002064. doi: 10.1002/14651858.CD002064.pub2. PMID: 12137643 Updated.
-
Treatment for sialorrhea (excessive saliva) in people with motor neuron disease/amyotrophic lateral sclerosis.Cochrane Database Syst Rev. 2022 May 20;5(5):CD006981. doi: 10.1002/14651858.CD006981.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593746 Free PMC article.
-
Gamma aminobutyric acid (GABA) modulators for amyotrophic lateral sclerosis/motor neuron disease.Cochrane Database Syst Rev. 2017 Jan 9;1(1):CD006049. doi: 10.1002/14651858.CD006049.pub2. Cochrane Database Syst Rev. 2017. PMID: 28067943 Free PMC article.
-
Mechanical ventilation for amyotrophic lateral sclerosis/motor neuron disease.Cochrane Database Syst Rev. 2017 Oct 6;10(10):CD004427. doi: 10.1002/14651858.CD004427.pub4. Cochrane Database Syst Rev. 2017. PMID: 28982219 Free PMC article.
Cited by
-
Discovery of Novel Inhibitors against ALS-Related SOD1(A4V) Aggregation through the Screening of a Chemical Library Using Differential Scanning Fluorimetry (DSF).Pharmaceuticals (Basel). 2024 Sep 27;17(10):1286. doi: 10.3390/ph17101286. Pharmaceuticals (Basel). 2024. PMID: 39458929 Free PMC article.
-
Association of Insulin-like Growth Factor 1 Concentrations with Risk for and Prognosis of Amyotrophic Lateral Sclerosis - Results from the ALS Registry Swabia.Sci Rep. 2020 Jan 20;10(1):736. doi: 10.1038/s41598-020-57744-x. Sci Rep. 2020. PMID: 31959864 Free PMC article.
-
More than a bystander: the contributions of intrinsic skeletal muscle defects in motor neuron diseases.Front Physiol. 2013 Dec 18;4:356. doi: 10.3389/fphys.2013.00356. Front Physiol. 2013. PMID: 24391590 Free PMC article. Review.
-
Multidisciplinary Interventions in Motor Neuron Disease.J Neurodegener Dis. 2014;2014:435164. doi: 10.1155/2014/435164. Epub 2014 Nov 18. J Neurodegener Dis. 2014. PMID: 26317009 Free PMC article. Review.
-
Transplanted modified muscle progenitor cells expressing a mixture of neurotrophic factors delay disease onset and enhance survival in the SOD1 mouse model of ALS.J Mol Neurosci. 2015 Mar;55(3):788-97. doi: 10.1007/s12031-014-0426-0. Epub 2014 Oct 21. J Mol Neurosci. 2015. PMID: 25330859
References
References to studies included in this review
Borasio 1998 {published data only}
-
- Borasio GD, Robberecht W, Leigh PN, Emile J, Guiloff RJ, Jerusalem F, et al. A placebo controlled trial of insulin‐like nerve growth factor‐1 in amyotrophic lateral sclerosis. Neurology 1998;51(2):583‐6. [PUBMED: 9710040] - PubMed
Lai 1997 {published data only}
-
- Lai EC, Felice KJ, Festoff BW, Gawel MJ, Gelinas DF, Kratz R, et al. Effect of recombinant human insulin‐like growth factor‐1 on progression of ALS. A placebo‐controlled study. Neurology 1997;49(6):1621‐30. [PUBMED: 9409357] - PubMed
References to studies excluded from this review
Nagano 2005 {published data only}
-
- Nagano I, Shiote M, Murakami T, Kamada H, Hamakawa I, Matsubara E, et al. Beneficial effects of intrathecal IGF‐I administration in patients with amyotrophic lateral sclerosis. Neurological Research 2005;27(7):768‐72. - PubMed
Additional references
Adem 1994
-
- Adem A, Ekblom J, Gillberg P‐G, Jossan SS, Hoog A, Winblad B, et al. Insulin‐like growth factor‐1 receptors in human spinal cord: changes in amyotrophic lateral sclerosis. Journal of Neural Transmission. General Section 1994;97(1):73‐84. - PubMed
Appel 1987
-
- Appel V, Stewart SS, Smith G, Appel SH. A rating scale for amyotrophic lateral sclerosis: description and preliminary experience. Annals of Neurology 1987;22(3):328‐33. - PubMed
Bharucha 1983
-
- Bharucha NE, Schoenberg BS, Raven RH, Pickle IW, Byar D, Mason TJ. Geographic distribution of motor neuron disease and correlation with possible etiologic factors. Neurology 1983;33(7):911‐5. - PubMed
Caroni 1990
Caroni 1993
-
- Caroni P. Activity‐sensitive signals by muscle‐derived IGFs in the developing and regenerating neuromuscular system. Annals of the New York Academy of Science 1993;692:209‐22. - PubMed
Dore 1996
-
- Dore S, Krieger C, Kar S, Quirion R. Distribution and levels of insulin like growth factor (IGF‐I and IGF‐II) and insulin receptor binding sites in the spinal cords of amyotrophic lateral sclerosis (ALS) patients. Brain Research. Molecular Brain Research 1996;41(1‐2):128‐33. - PubMed
GRADE 2004
Hantai 1995
-
- Hantai D, Akaabourne M, Lagord C, Murawsky, Houenou LJ, Festoff BW, et al. Beneficial effects of insulin‐like growth factor‐I on wobbler mouse motoneuron disease. Journal of the Neurological Sciences 1995;129(Suppl):122‐6. - PubMed
Haverkamp 1995
-
- Haverkamp LJ, Appel V, Appel SH. Natural history of amyotrophic lateral sclerosis in a database population ‐ validation of a scoring system and a model for survival prediction. Brain 1995;118(3):707‐19. - PubMed
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Kaspar 2003
-
- Kaspar BK, Llado J, Sherkat N, Rothstein JD, Gage FH. Retrograde viral delivery of IGF‐I prolongs survival in a mouse ALS model. Science 2003;301:839‐842. - PubMed
Lewis 1993
-
- Lewis ME, Neff NT, Contreras PC, Strong DB, Oppenheim RW, Grebow PE, et al. Insulin‐like growth factor‐1: potential for treatment of motor neuronal disorder. Experimental Neurology 1993;124(1):73‐88. - PubMed
McGuire 1996
-
- McGuire D, Garrison L, Miller RG. Relationship of the Tufts Quantitative Neuromuscular Exam (TQNE) and the Sickness Impact Profile (SIP) in measuring progression of ALS. Neurology 1996;46(5):1442‐4. - PubMed
Miller 1999
-
- Miller RG, Munsat TL, Swash M, Brooks BR. Consensus guidelines for the design and implementation of clinical trials in ALS. Journal of the Neurological Sciences 1999;169(1‐2):2‐12. - PubMed
Mitchell 1998
Neff 1993
-
- Neff NT, Prevette D, Houenou LJ, Lewis ME, Glicksman MA, Yin QW, et al. Insulin‐like growth factors: putative muscle‐derived trophic agents that promote motoneuron survival. Journal of Neurobiology 1993;24(12):1558‐66. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

