Macrolide antibiotics for cystic fibrosis
- PMID: 23152214
- PMCID: PMC7098459
- DOI: 10.1002/14651858.CD002203.pub4
Macrolide antibiotics for cystic fibrosis
Update in
-
Macrolide antibiotics (including azithromycin) for cystic fibrosis.Cochrane Database Syst Rev. 2024 Feb 27;2(2):CD002203. doi: 10.1002/14651858.CD002203.pub5. Cochrane Database Syst Rev. 2024. PMID: 38411248 Free PMC article.
Abstract
Background: Macrolide antibiotics may have a modifying role in diseases which involve airway infection and inflammation, like cystic fibrosis.
Objectives: To test the hypotheses that, in people with cystic fibrosis, macrolide antibiotics: 1. improve clinical status compared to placebo or another antibiotic; 2. do not have unacceptable adverse effects. If benefit was demonstrated, we aimed to assess the optimal type, dose and duration of macrolide therapy.
Search methods: We searched the Cochrane Cystic Fibrosis and Genetic Disorders Group Trials Register comprising references identified from comprehensive electronic database searches, handsearching relevant journals and abstract books of conference proceedings.We contacted investigators known to work in the field, previous authors and pharmaceutical companies manufacturing macrolide antibiotics for unpublished or follow-up data (May 2010).Latest search of the Group's Cystic Fibrosis Trials Register: 29 February 2012.
Selection criteria: Randomised controlled trials of macrolide antibiotics compared to: placebo; another class of antibiotic; another macrolide antibiotic; or the same macrolide antibiotic at a different dose.
Data collection and analysis: Two authors independently extracted data and assessed risk of bias. Seven groups were contacted and provided additional data which were incorporated into the review.
Main results: Ten of 31 studies identified were included (959 patients). Five studies with a low risk of bias examined azithromycin versus placebo and demonstrated consistent improvement in forced expiratory volume in one second over six months (mean difference at six months 3.97% (95% confidence interval 1.74% to 6.19%; n = 549, from four studies)). Patients treated with azithromycin were approximately twice as likely to be free of pulmonary exacerbation at six months, odds ratio 1.96 (95% confidence interval 1.15 to 3.33). With respect to secondary outcomes, there was a significant reduction in need for oral antibiotics and greater weight gain in those taking azithromycin. Adverse events were uncommon and not obviously associated with azithromycin, although a once-weekly high dose regimen was associated with more frequent gastrointestinal adverse events. Treatment with azithromycin was associated with reduced identification of Staphylococcus aureus on respiratory culture, but also a significant increase in macrolide resistance.
Authors' conclusions: This review provides evidence of improved respiratory function after six months of azithromycin. Data beyond six months were less clear, although reduction in pulmonary exacerbation was sustained. Treatment appeared safe over a six-month period; however, emergence of macrolide resistance was a concern. A multi-centre trial examining long-term effects of this antibiotic treatment is needed, especially for infants recognised through newborn screening.
Conflict of interest statement
None known.
Figures





















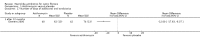











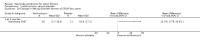









Update of
-
Macrolide antibiotics for cystic fibrosis.Cochrane Database Syst Rev. 2011 Dec 7;(12):CD002203. doi: 10.1002/14651858.CD002203.pub3. Cochrane Database Syst Rev. 2011. Update in: Cochrane Database Syst Rev. 2012 Nov 14;11:CD002203. doi: 10.1002/14651858.CD002203.pub4. PMID: 22161368 Updated.
References
References to studies included in this review
Clement 2006 {published and unpublished data}
-
- Clement A, Tamalet A, Roux E, Ravilly S, Jais JP. Long term effects and clinical outcome of low‐dose azithromycin in young patients with cystic fibrosis: a mulicenter, randomized, double‐blind, placebo‐controlled trial [abstract]. Journal of Cystic Fibrosis 2005;4(Suppl1):S68.
-
- Clement A, Tamalet A, Leroux E, Ravilly S, Jais J. Long term effects and clinical outcome of low‐dose azithromycin in young patients with cystic fibrosis: a multicenter, randomized, double‐blind, placebo‐controlled trial [abstract]. Pediatric Pulmonology 2005;40(Suppl 28):285.
Equi 2002 {published and unpublished data}
-
- Equi A, Balfour‐Lynn IM, Bush A, Rosenthal M. Long term azithromycin in children with cystic fibrosis: a randomised, placebo‐controlled crossover trial. Lancet 2002;360(9338):978‐84. - PubMed
-
- Equi A, Bush A, Alton EW, Balfour‐Lynn I, Rosenthal M. A prospective, double‐blind, randomised, placebo controlled, crossover trial of long term azithromycin in children [abstract]. Pediatric Pulmonology 2001;Suppl 22:307.
-
- Equi A, Bush A, Balfour‐Lynn IM. A prospective, double‐blind, randomised, placebo controlled, crossover trial of azithromycin in paediatric cystic fibrosis [abstract]. Thorax 2002;Suppl 3:iii38. - PubMed
Kabra 2010 {published and unpublished data}
-
- Kabra SK, Pawaiya R, Lodha R, Kapil A, Kabra M, Vani AS, et al. Long‐term daily high and low doses of azithromycin in children with cystic fibrosis: a randomized controlled trial. Journal of Cystic Fibrosis 2010;9(1):17‐23. - PubMed
McCormack 2007 {published data only}
-
- Bell S, Serisier D, Wainwright C, Bowler S, Senini S, Walmsley K, et al. 6‐month treatment with azithromycin reduces hospital admissions for respiratory exacerbations in cystic fibrosis [abstract]. Respirology 2006; Vol. 11, issue Suppl 2:A58.
-
- Bell S, Serisier D, Wainwright C, Bowler S, Senini S, Walmsley K, et al. Randomised, prospective double‐blind trial of long term daily versus weekly azithromycin in cystic fibrosis [abstract]. Respirology 2006; Vol. 11, issue Suppl 2:A11.
-
- McCormack J, Bell S, Senini S, Walmsley K, Patel K, Wainwright C, et al. Daily versus weekly azithromycin in cystic fibrosis patients. European Respiratory Journal 2007;30(3):487‐95. - PubMed
-
- Senini S, McCormack J. A randomised, prospective double‐blind trial of long‐term daily versus weekly azithromycin in cystic fibrosis. Australian New Zealand Clinical Trials Registry (www.anzctr.org.au) (accessed 04 March 2010) 2005.
O'Connor 2009 {published and unpublished data}
-
- O'Connor BS. The use of azithromycin in cystic fibrosis patients not infected with Pseudomonas aeruginosa. Belfast, UK: Faculty of Medicine and Health Sciences, The Queen's University, 2009.
-
- O'Connor BS, Reid AL, Steen HJ, Shields MD, Elborn JS. Use of azithromycin in cystic fibrosis patients not infected with Pseudomonas aeruginosa [abstract]. Journal of Cystic Fibrosis 2008;7(Suppl 2):S25.
Rotschild 2005 {published data only}
-
- Rotschild M, Elias N, Berkowitz D, Pollak S, Shinawi M, Beck R, Bentur L. Autoantibodies against bactericidal/permeability‐increasing protein (BPI‐ANCA) in cystic fibrosis patients treated with azithromycin. Clinical and Experimental Medicine 2005;5(2):80‐5. - PubMed
Saiman 2003 {published and unpublished data}
-
- Nguyen D, Mayor‐Hamblett N, Marshall BC, Saiman L, Burns JL. Phenotypic characterization of pseudomonas aeruginosa as a potential predictor of clinical response to azithromycin in CF patients [abstract]. Pediatric Pulmonology 2004;38(Suppl 27):286.
-
- Saiman L. What have we learned from further analysis of the U.S. Macrolide Trial?: Subgroup analysis of azithromycin trial [abstract]. Pediatric Pulmonology 2003;36 Suppl 25:165‐7.
-
- Saiman L, Marshall BC, Mayer‐Hamblett N, Burns JL, Quittner AL, Cibene DA, et al. Azithromycin in patients with cystic fibrosis chronically infected with Pseudomonas aeruginosa: a randomized controlled trial. JAMA 2003;290(13):1749‐56. - PubMed
-
- Saiman L, Mayer‐Hamblett N, Campbell P, Marshall BC. Heterogeneity of treatment response to azithromycin in patients with cystic fibrosis. American Journal of Respiratory and Critical Care Medicine 2005;172(8):1008‐12. - PubMed
Saiman 2010 {published and unpublished data}
-
- Anstead M, Saiman L, Mayer‐Hamblett N, Lands LC, Kloster M, Goss CH, et al. Pulmonary exacerbations in patients 6‐18 years old with CF and mild lung disease uninfected with pseudomonas aeruginosa [abstract]. Pediatric Pulmonology 2010;45(Suppl 33):377, Abstract no: 440. [CFGD Register: MA24f]
-
- Ratjen F, Saiman L, Mayer‐Hamblett N, Lands LC, Kloster M, Emmett P, et al. The effect of azithromycin on inflammatory markers in CG children and adolescents uninfected with pseudomonas aeruginosa [abstract]. Pediatric Pulmonology 2010;45(Suppl 33):271, Abstract no:149. [CFGD Register : MA24e]
-
- Saiman L. Azithromycin in CF patients uninfected with pseudomonas aeruginosa [abstract]. Pediatric Pulmonology 2009;44(32):185. [CFGD Register: MA24a]
-
- Saiman L, Anstead M, Mayer‐Hamblett N, Lands LC, Kloster M, Hocevar‐Trnka J, et al. Effect of azithromycin on pulmonary function in patients with cystic fibrosis uninfected with Pseudomonas aeruginosa: a randomized controlled trial. JAMA 2010;303(17):1707‐15. [CFGD Register: MA24c] - PubMed
Steinkamp 2007 {published and unpublished data}
-
- Steinkamp G, Schmitt‐Grohe S, Doring G, Staab D, Pfrunder D, Beck G, et al. Once‐weekly azithromycin in cystic fibrosis with chronic Pseudomonas aeruginosa infection. Respiratory Medicine 2008;102(11):1643‐53. - PubMed
-
- Steinkamp G, Schmitt‐Grohe S, Doring G, Stabb D, Schubert R, Zielen S. Once weekly azithromycin in cystic fibrosis: a double‐blind, randomised trial in patients with chronic pseudomonas aeruginosa infection [abstract]. Pediatric Pulmonology 2007;42(Suppl 30):300.
-
- Steinkamp G, Schmitt‐Grohe S, Doring G, Worlitzsch D, Staab D, Schubert R, et al. Clinical and immunomodulatory effects of once weekly azithromycin treatment in cystic fibrosis patients chronically infected with pseudomonas aeruginosa [abstract]. Journal of Cystic Fibrosis 2006;5(Suppl):S25.
Wolter 2002 {published data only}
-
- Bell SC, Seeney S, Walmsley K, Wolter JM, Bowler SD, McCormack JG. Long‐term treatment with azithromycin results in reduced ex‐vivo inflammatory cytokine production in adults with cystic fibrosis [abstract]. Pediatric Pulmonology 2002;34 Suppl 24:289‐90.
-
- Bowler SD. Effect of long‐term treatment with azithromycin on disease parameters in cystic fibrosis [abstract]. Japanese Journal of Antibiotics 2003;56(Suppl A):38. - PubMed
-
- Bowler SD, Masel PJ, Bell SC, Seeney SL, Wolter JM, McCormack JG. A prospective, randomised trial of long term azithromycin (AZM) versus placebo in cystic fibrosis; impact on clinical, laboratory and quality of life (QOL) outcomes [abstract]. Pediatric Pulmonology 2000;30 Suppl 20:280.
-
- Bowler SD, Seeney S, Walmsley K, Wolter JM, Bell SC, McCormack JG. Long term treatment with azithromycin results in reduced in vitro inflammatory cytokine production in adults with cystic fibrosis [abstract]. Respirology 2002;7 Suppl 1:A9.
-
- Seeney SL, Bowler SD, Wolter JM, Bell SC, Masel PJ, McCormack JG. A prospective randomised trial of long term azithromycin (AZM) versus placebo in cystic fibrosis (CF): impact on clinical, laboratory and quality of life (QOL) outcomes [abstract]. Internal Medicine Journal 2001;31 Suppl:A12.
References to studies excluded from this review
Aaron 2005 {published data only}
-
- Aaron S. Clinical evidence for combination antibiotic susceptibility testing (synergy testing) [abstract]. Pediatric Pulmonology 2008;43(Suppl 1):157.
-
- Aaron S, Vandemheen K, Ferris W, Tullis E, Haase D, Berthiaume Y, et al. Treatment of CF exacerbations based on multiple combination antibiotic susceptibility testing‐a randomized, double‐blind, controlled clinical trial [abstract]. Pediatric Pulmonology 2005;40(Suppl 28):304.
-
- Aaron SD, Vandemheen KL, Ferris W, Fergusson D, Tullis E, Haase D, et al. Combination antibiotic susceptibility testing to treat exacerbations of cystic fibrosis associated with multiresistant bacteria: a randomised, double‐blind, controlled clinical trial. Lancet 2005;366(9484):463‐71. - PubMed
Anstead 1999 {published data only}
-
- Anstead MI, Kuhn RJ, Hartford LH, Craigmyle L, Halsey S, Kanga JF. Effect of chronic azithromycin on lung function in cystic fibrosis. Pediatric Pulmonology 1999;28 Suppl 19:283‐4.
Anstead 2001 {published data only}
-
- Anstead M, Kuhn RJ, Halsey S, Doherty DE, D'Souza N, Kanga JF. Effect of azithromycin on lung function, sputum bacteriology, and sputum inflammatory markers in cystic fibrosis [abstract]. American Journal of Respiratory and Critical Care Medicine 2001;163(5 Suppl):A565.
Baumann 2000 {published data only}
-
- App EM, Konig A, Duffner K, Baumann U, King M, Hardt H. The effects of azithromycin therapy on sputum inflammation in CF lung disease [abstract]. American Journal of Respiratory and Critical Care Medicine 2000;161(3 Suppl):A758.
-
- Baumann U, App E, King M, Fischer J, Zimmermann T, Sextro W, et al. Low‐dose azithromycin therapy reaches sputum drug levels of potential antipseudomonal activity in cystic fibrosis patients [abstract]. Journal of Cystic Fibrosis 2002;1(Suppl 1):S130.
-
- Baumann U, App EM, Konig A, Sextro W, Matthys H, Hardt H. Sputum DNA under long‐term therapy with azithromycin [abstract]. Proceedings of the 13th International Cystic Fibrosis Congress; 2000 June 4‐8; Stockholm. 2000:164.
-
- Baumann U, Fischer JJ, Tummler B, Sextro W, App EM, King M, et al. Long‐term low‐dose therapy with azithromycin in CF [abstract]. Proceedings of the 13th International Cystic Fibrosis Congress;2000 June 4‐8; Stockholm. 2000:165.
Beringer 2005 {published data only}
-
- Beringer P, Kriengkauykiat J, Louie S, Gill M, Han E, Woo M, et al. Controlled pharmacokinetic study of azithromycin in adult cystic fibrosis patients [abstract]. Pediatric Pulmonology 2004;38(Suppl 27):285.
Cipolli 2001 {published data only}
-
- Cipolli M, Cazzola G, Novelli A, Cassetta MI, Fallani S, Mazzei T. Azithromycin concentrations in serum and bronchial secretions of patients with cystic fibrosis. Clinical Drug Investigation 2001;21(5):353‐60.
Dionyssopoulou 2005 {published data only}
-
- Dionyssopoulou V, Perpati G, Stefanatou E, Christodoulou E, Kalatzi E, Armeniakou E, et al. Laboratory and clinical effect of long‐term roxithromycin treatment in adult patients with cystic fibrosis [abstract]. Journal of Cystic Fibrosis 2005;4(Suppl 1):S24.
Dogru 2004 {published data only}
-
- Dogru D, Dalgic F, Kiper N, Ozcelik U, Yalcin E, Aslan AT, et al. Effect of clarithromycin on inflammatory markers in bronchoalveolar lavage fluid in cystic fibrosis: a randomised, placebo‐controlled crossover trial [abstract]. Journal of Cystic Fibrosis 2004;3(Suppl 1):S21.
Frederiksen 2001 {published and unpublished data}
-
- Frederiksen B, Koch C, Hoiby N, Pressler T. Clinical efficacy of clarithromycin in CF patients with chronic lung infection [abstract]. Abstracts of the 24th European Cystic Fibrosis Conference; 2001 June 6‐9; Vienna. 2001:P208.
Jaffe 1998 {published data only}
-
- Jaffe A, Francis J, Rosenthal M, Bush A. Long‐term azithromycin may improve lung function in children with cystic fibrosis. Lancet 1998;351(9100):420. - PubMed
Jensen 2005 {published data only}
-
- Jensen PØ, Moser C, Pressler T, Koch C, Høiby N. Circulating phagocytes during Azithromycin treatment of Cystic Fibrosis patients with chronic Pseudomonas aeruginosa lung infection [abstract]. Journal of Cystic Fibrosis 2005;4(Suppl 1):S24.
Kessaris 2003 {published data only}
-
- Kessaris A, Souli M, Inglezos I, Kanellakopoulou K, Apostolopoulou F, Giamarellou E. Efficacy of low‐dose, long‐term clarithromycin administration in improving the clinical course in cystic fibrosis patients with chronic P. aeruginosa airway colonization [abstract]. European Respiratory Journal 2003;22(Suppl 45):514s.
Ordonez 2001 {published data only}
-
- Ordonez CL, Stulbarg M, Grundland H, Liu JT, Boushey HA. Effect of clarithromycin on airway obstruction and inflammatory markers in induced sputum in cystic fibrosis: a pilot study. Pediatric Pulmonology 2001;32(1):29‐37. - PubMed
Pirzada 1999 {published and unpublished data}
-
- Pirzada, OM Taylor, CJ. Long term macrolide antibiotics improve pulmonary function in cystic fibrosis [abstract]. Pediatric Pulmonology. 1999; Vol. 28 Suppl 19:263.
Pukhalsky 2001 {published data only}
-
- Pukhalsky AL, Shmarina GV, Kaproanov NI, Kashirskaja NJ, Kokarovtseva SN, Shabalova LA, et al. Increase of the sputum neutrophil elastase activity is a paradoxical effect of the successful lung disease treatment in cystic fibrosis [abstract]. Pediatric Pulmonology 2001;32 Suppl 22:274.
Radionovitch 2005 {published and unpublished data}
-
- Radionovitch AM, Kashirskaya NJ, Kapranov NI. Long‐term low‐dose therapy with macrolides in bronchopulmonary disease in Cystic Fibrosis (CF) pediatric patients [abstract]. Journal of Cystic Fibrosis 2005;4(Suppl 1):S31.
Rubin 2003 {published data only}
-
- Barker PM, Gillie DJ, Schechter MS, Rubin BK. Effect of macrolides on in vivo ion transport across cystic fibrosis nasal epithelium. American Journal of Respiratory and Critical Care Medicine 2005;171(8):868‐71. - PubMed
-
- Rubin BK. Macrolide antibiotic therapy for patients with cystic fibrosis. www.clinicaltrials.gov (accessed 04 March 2010) 2005.
Shmarina 2004 {published data only}
-
- Shmarina GV, Pukhalsky AL, Kashirskaja NJ, Research Centre for Medical Genetics MR. Anti‐inflammatory therapy in cystic fibrosis: a comparative study in the treatment with nimesulide (selective cyclooxygenase‐2 inhibitor) and clarithromycin (14‐memebered ring macrolide antibiotic) [abstract]. European Respiratory Journal 2004;24(Suppl 48):P3758.
Sriram 2003 {published and unpublished data}
-
- Sriram S, Young J, Waterhouse JC, Bucknall CE, Stack BHR. The antiinflammatory effect of clarithromycin in CF [abstract]. Journal of Cystic Fibrosis 2003;2(Suppl 1):S52.
References to studies awaiting assessment
Elmasry 2010 {published data only}
-
- Elmasry S, Mok SS, Braithwaite M, Sofianopoulos S, Clark D, Finlayson F, et al. Adherence behaviour of adult cystic fibrosis (CF) patients to prescribed azithromycin [abstract]. Journal of Cystic Fibrosis 2010;9(Suppl 1):S24, Abstract no: 92.
Pukhalsky 2008 {published data only}
-
- Pukhalsky A, Shmarina G, Pukhalskaya D, Perederko L. Whether low frequency of hepatobiliary abnormalities in cystic fibrosis patients is associated with anti‐inflammatory treatment? [abstract]. European Respiratory Society Annual Congress; 2008 Oct 4‐8; Berlin, Germany. 2008:216s.
Additional references
Anderson 1996
-
- Anderson R, Theron AJ, Feldman C. Membrane‐stabilizing, anti‐inflammatory interactions of macrolides with human neutrophils. Inflammation 1996;20(6):693‐705. - PubMed
Ball 1991
-
- Ball AP. Azithromycin: an interim analysis. Journal of International Medical Research 1991;19(6):446‐50. - PubMed
Boucher 1999
Curtin 2002a
Curtin 2002b
Curtin 2002c
Florescu 2009
-
- Florescu DF, Murphy PJ, Kalil AC. Effects of prolonged use of azithromycin in patients with cystic fibrosis: a meta‐analysis. Pulmonary Pharmacology and Therapeutics 2009;22(6):467‐72. - PubMed
Hansen 2009
-
- Hansen CR, Pressler T, Hoiby N, Johansen HK. Long‐term, low‐dose azithromycin treatment reduces the incidence but increases macrolide resistance in Staphylococcus aureus in Danish CF patients. Journal of Cystic Fibrosis 2009;8(1):58‐62. - PubMed
Higgins 2003
Higgins 2009
-
- Higgins JPT, Altman DG on behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 [updated September 2009]. The CochraneCollaboration, 2008. Available from www.cochrane‐handbook.org.
Hoiby 1994
Hutchison 1999
-
- Hutchison ML, Govan JR. Pathogenicity of microbes associated with cystic fibrosis. Microbes and Infection 1999;1(12):1005‐14. - PubMed
Ichimiya 1996
-
- Ichimiya T, Takeoka K, Hiramatsu K, Hirai K, Yamasaki T, Nasu M. The influence of azithromycin on the biofilm formation of Pseudomonas aeruginosa in vitro. Chemotherapy 1996;42(3):186‐91. - PubMed
Kobayashi 1993
-
- Kobayashi H, Ohgaki N, Takeda H. Therapeutic possibilities for diffuse panbronchiolitis. International Journal of Antimicrobial Agents 1993;3:S81‐S86. - PubMed
Labro 1998
-
- Labro MT. Anti‐inflammatory activity of macrolides: a new therapeutic potential?. Journal Antimicrobial Chemotherapeutics 1998;41 Suppl B:37‐46. - PubMed
Matsui 1998
-
- Matsui H, Grubb BR, Tarran R, Randell SH, Gatzy JT, Davis CW, et al. Evidence for periciliary liquid layer depletion, not abnormal ion composition in the pathogenesis of cystic fibrosis airways disease. Cell 1998;95(7):1005‐15. - PubMed
Mizukane 1994
Molinari 1993
-
- Molinari G, Guzman CA, Pesce A, Schito GC. Inhibition of Pseudomonas aeruginosa virulence factors by subinhibitory concentrations of azithromycin and other macrolide antibiotics. Journal of Antimicrobial Chemotherapy 1993;31(5):681‐8. - PubMed
Phaff 2006
-
- Phaff SJ, Tiddens HA, Verbrugh HA, Ott A. Macrolide resistance of Staphylococcus aureus and Haemophilus species associated with long‐term azithromycin use in cystic fibrosis. Journal of Antimicrobial Chemotherapy 2006;57(4):741‐6. - PubMed
Ratjen 2012
Retsema 1987
RevMan 2008 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.0. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2008.
Riordan 1989
-
- Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science 1989;245(4922):1066‐73. - PubMed
Smyth 2003
Steinkamp 2010
-
- Steinkamp G (CF‐Centre Hamburg‐Altona and Clinical Research Hannover, Germany). Email to KW Southern 2010.
Tramper‐Stranders 2007
-
- Tramper‐Stranders GA, Wolfs TF, Fleer A, Kimpen JL, Ent CK. Maintenance azithromycin treatment in pediatric patients with cystic fibrosis: long‐term outcomes related to macrolide resistance and pulmonary function. Pediatric Infectious Disease Journal 2007;26(1):8‐12. - PubMed
Yanagihara 1997
-
- Yanagihara K, Tomono K, Sawai T, Hirakata Y, Kadota J, Koga H, et al. Effect of clarithromycin on lymphocytes in chronic respiratory Pseudomonas aeruginosa infection. American Journal of Respiratory and Critical Care Medicine 1997;155(1):337‐42. - PubMed
References to other published versions of this review
Southern 2003
Southern 2004
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

