Selective serotonin reuptake inhibitors (SSRIs) for stroke recovery
- PMID: 23152272
- PMCID: PMC6465036
- DOI: 10.1002/14651858.CD009286.pub2
Selective serotonin reuptake inhibitors (SSRIs) for stroke recovery
Update in
-
Selective serotonin reuptake inhibitors (SSRIs) for stroke recovery.Cochrane Database Syst Rev. 2019 Nov 26;2019(11):CD009286. doi: 10.1002/14651858.CD009286.pub3. Cochrane Database Syst Rev. 2019. Update in: Cochrane Database Syst Rev. 2021 Nov 15;11:CD009286. doi: 10.1002/14651858.CD009286.pub4. PMID: 31769878 Free PMC article. Updated.
Abstract
Background: Stroke is the major cause of adult disability. Selective serotonin reuptake inhibitors (SSRIs) have been used for many years to manage depression. Recently, small trials have demonstrated that SSRIs might improve recovery after stroke, even in people who are not depressed. Systematic reviews and meta-analyses are the least biased way to bring together data from several trials. Given the promising effect of SSRIs on stroke recovery seen in small trials, a systematic review and meta-analysis is needed.
Objectives: To determine whether SSRIs improve recovery after stroke, and whether treatment with SSRIs was associated with adverse effects.
Search methods: We searched the Cochrane Stroke Group Trials Register (August 2011), Cochrane Depression Anxiety and Neurosis Group Trials Register (November 2011), Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2011, Issue 8), MEDLINE (from 1948 to August 2011), EMBASE (from 1980 to August 2011), CINAHL (from 1982 to August 2011), AMED (Allied and Complementary Medicine) (from 1985 to August 2011), PsycINFO (from 1967 to August 2011) and PsycBITE (Pyschological Database for Brain Impairment Treatment Efficacy) (March 2012). To identify further published, unpublished and ongoing trials we searched trials registers, pharmaceutical websites, reference lists, contacted experts and performed citation tracking of included studies.
Selection criteria: We included randomised controlled trials that recruited stroke survivors (ischaemic or haemorrhagic) at any time within the first year. The intervention was any SSRI, given at any dose, for any period. We excluded drugs with mixed pharmacological effects. The comparator was usual care or placebo. In order to be included, trials had to collect data on at least one of our primary (dependence and disability) or secondary (impairments, depression, anxiety, quality of life, fatigue, healthcare cost, death, adverse events and leaving the trial early) outcomes.
Data collection and analysis: We extracted data on demographics, type of stroke, time since stroke, our primary and secondary outcomes, and sources of bias. For trials in English, two review authors independently extracted data. For Chinese papers, one review author extracted data. We used standardised mean differences (SMD) to estimate treatment effects for continuous variables, and risk ratios (RR) for dichotomous effects, with their 95% confidence intervals (CIs).
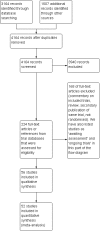
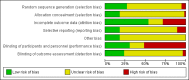
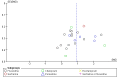
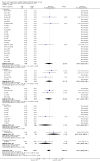
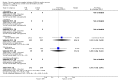
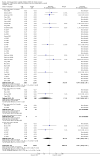
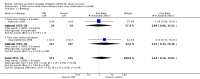
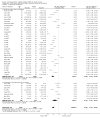
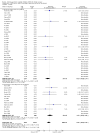
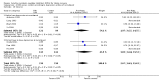
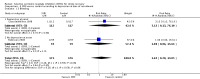
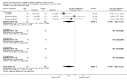
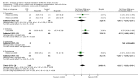
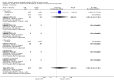
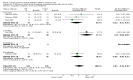
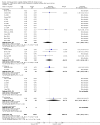
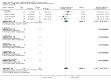
Main results: We identified 56 completed trials of SSRI versus control, of which 52 trials (4059 participants) provided data for meta-analysis. There were statistically significant benefits of SSRI on both of the primary outcomes: RR for reducing dependency at the end of treatment was 0.81 (95% CI 0.68 to 0.97) based on one trial, and for disability score, the SMD was 0.91 (95% CI 0.60 to 1.22) (22 trials involving 1343 participants) with high heterogeneity between trials (I(2) = 87%; P < 0.0001). For neurological deficit, depression and anxiety, there were statistically significant benefits of SSRIs. For neurological deficit score, the SMD was -1.00 (95% CI -1.26 to -0.75) (29 trials involving 2011 participants) with high heterogeneity between trials (I(2) = 86%; P < 0.00001). For dichotomous depression scores, the RR was 0.43 (95% CI 0.24 to 0.77) (eight trials involving 771 participants) with high heterogeneity between trials (I(2) = 77%; P < 0.0001). For continuous depression scores, the SMD was -1.91 (95% CI -2.34 to -1.48) (39 trials involving 2728 participants) with high heterogeneity between trials (I(2) = 95%; P < 0.00001). For anxiety, the SMD was -0.77 (95% CI -1.52 to -0.02) (eight trials involving 413 participants) with high heterogeneity between trials (I(2) = 92%; P < 0.00001). There was no statistically significant benefit of SSRI on cognition, death, motor deficits and leaving the trial early. For cognition, the SMD was 0.32 (95% CI -0.23 to 0.86), (seven trials involving 425 participants) with high heterogeneity between trials (I(2) = 86%; P < 0.00001). The RR for death was 0.76 (95% CI 0.34 to 1.70) (46 trials involving 3344 participants) with no heterogeneity between trials (I(2) = 0%; P = 0.85). For motor deficits, the SMD was -0.33 (95% CI -1.22 to 0.56) (two trials involving 145 participants). The RR for leaving the trial early was 1.02 (95% CI 0.86 to 1.21) in favour of control, with no heterogeneity between trials. There was a non-significant excess of seizures (RR 2.67; 95% CI 0.61 to 11.63) (seven trials involving 444 participants), a non-significant excess of gastrointestinal side effects (RR 1.90; 95% CI 0.94 to 3.85) (14 trials involving 902 participants) and a non-significant excess of bleeding (RR 1.63; 95% CI 0.20 to 13.05) (two trials involving 249 participants) in those allocated SSRIs. Data were not available on quality of life, fatigue or healthcare costs.There was no clear evidence from subgroup analyses that one SSRI was consistently superior to another, or that time since stroke or depression at baseline had a major influence on effect sizes. Sensitivity analyses suggested that effect sizes were smaller when we excluded trials at high or unclear risk of bias.Only eight trials provided data on outcomes after treatment had been completed; the effect sizes were generally in favour of SSRIs but CIs were wide.
Authors' conclusions: SSRIs appeared to improve dependence, disability, neurological impairment, anxiety and depression after stroke, but there was heterogeneity between trials and methodological limitations in a substantial proportion of the trials. Large, well-designed trials are now needed to determine whether SSRIs should be given routinely to patients with stroke.
Conflict of interest statement
Gillian Mead, Maree Hackett and Graeme Hankey are co‐principal investigators on the planned FOCUS trial (Fluoxetine or control under supervision) in the UK and the AFFINITY (Assessment of fluoxetine in stroke recovery) trial in Australia designed to assess the impact of fluoxetine on disability and dependency after stroke. These trials fulfil our inclusion criteria.
Figures




































































































Comment in
-
ACP Journal Club. Review: SSRIs somewhat improve dependence and disability after stroke.Ann Intern Med. 2013 Mar 19;158(6):JC12. doi: 10.7326/0003-4819-158-6-201303190-02012. Ann Intern Med. 2013. PMID: 23553006 No abstract available.
References
References to studies included in this review
-
- Acler M, Avesani A, Fiaschi A, Manganotti P. Serotonergic modulation of brain excitability and motor recovery in patients affected by stroke. A double blind placebo RCT. International Journal of Stroke 2008;3:336.
- Acler M, Robol E, Fiaschi A, Manganotti P. A double blind placebo RCT to investigate the effects of serotonergic modulation on brain excitability and motor recovery in stroke patients. Journal of Neurology 2009;256:1152‐8. - PubMed
-
- Almeida OP, Waterreus A, Hankey GJ. Preventing depression after stroke: results from a randomized placebo‐controlled trial. Journal of Clinical Psychiatry 2006;67(7):1104‐9. - PubMed
-
- Andersen G, Vestergaard K, Lauritzen L. Effective treatment of post‐stroke depression with selective serotonin reuptake inhibitors. Journal of Neurology 1994;241:S42. - PubMed
- Andersen G, Vestergaard K, Lauritzen L. Effective treatment of post‐stroke depression with the selective serotonin reuptake inhibitor citalopram. Stroke 1994;25(6):1099‐104. - PubMed
- Andersen G, Vestergaard K, Lauritzen L. Post‐stroke depression treated with citalopram. Acta Neurologica Scandinavica 1994;89 Suppl 155:20.
- Andersen G, Vestergaard K, Lauritzen L. Post‐stroke depression treated with citalopram ‐ a selective serotonin reuptake inhibitor. Canadian Journal of Neurological Sciences 1993;20:S115.
- Andersen G, Vestergaard K, Lauritzen L. Post‐stroke depression treated with citalopram a selective serotonin reuptake inhibitor. Proceedings of the 7th Scandinavian Meeting on Cerebrovascular Disease. 1993:54.
- Andersen G, Vestergaard K, Lauritzen LU. Effective treatment of depression following apoplexy with citalopram. Ugeskrift for Laeger 1995;157(14):2000‐3. - PubMed
- Flicker C, Andersen G, Bayer L. A placebo controlled study of citalopram treatment for post‐stroke depression. Proceedings of the 11th Annual Meeting of the American Association for Geriatric Psychiatry. 1998.
-
- Brown KW, Sloan RL, Pentland B. Fluoxetine as a treatment for post‐stroke emotionalism. Acta Psychiatrica Scandinavica 1998;98(6):455‐8. - PubMed
-
- Burns A, Russell E, Stratton‐Powell H, Tyrell P, O'Neill P, Baldwin R. Sertraline in stroke‐associated lability of mood. International Journal of Geriatric Psychiatry 1999;14(8):681‐5. - PubMed
References to studies excluded from this review
-
- Berends HI, Nijlant JMM, Putten MJAM, Movig KLL, IJzerman MJ. Single dose of fluoxetine increases muscle activation in chronic stroke patients. Clinical Neuropharmacology 2009;32(1):1‐5. - PubMed
- Ijzerman MJ, Gendereren HI, Nijlant JJM. Effect of a single dose of the SSRI fluoxetine on motor activation patterns of the upper extremity in chronic stroke patients. Neurorehabilitation and Neural Repair 2006;20:158.
-
- Bian X, Zhu GZ, Liu Y, Hu XQ. Meclofenoxate combined with fluoxetine vs fluoxetine alone in treatment of depression after cerebral infarction. Chinese Journal of New Drugs and Clinical Remedies. 2006;25(5):372‐5.
-
- Chen SD. Role of fluoxetine hypochloride and clomipramine in ameliorating vascular depression and the side effects. Chinese Journal of Clinical Rehabilitation 2005;9(20):24‐5.
-
- Choi‐Kwon S, Choi J, Kwon SU, Kang DW, Kim JS. Fluoxetine improves the quality of life in patients with poststroke emotional disturbances. Cerebrovascular Diseases 2008;26(3):266‐71. - PubMed
-
- Cui DD. Depression associated with cerebral stroke. Hong Kong Medical Journal 2001;7(4):32.
References to studies awaiting assessment
-
- Sitzer M, Huff W, Steckel R. Prevention of poststroke depression after acute ischemic stroke using the selective serotonin reuptake‐inhibitor sertraline (PreDis‐study). Stroke 2002;33(2):651‐2.
-
- Whyte E. Sertraline for prevention post‐stroke depression and improving rehabilitation outcomes, 2005. www.clinicaltrials.gov (accessed 31 August 2012).
References to ongoing studies
-
- Anonymous. Influence of escitalopram on the incidence of depression and dementia following acute middle cerebral artery territory infarction. A randomised placebo‐controlled double blind study, 2006. http://www.clinicaltrialsregister.eu.
-
- Hankey G. Assessment of fluoxetine in stroke recovery (AFFINITY) trial, 2011. Australian New Zealand Clinical Trials Registry (ANZCTR) http://www.anzc... (accessed 31 August 2012).
-
- Carda S, Cisari C. Effects of clinical and functional outcome of escitalopram in adult stroke patients, 2009. www.clinicaltrials.gov (accessed 31 August 2012). [NCT00967408]
-
- Kim JS. The preventative effect of escitalopram on depression and related emotional disorders in acute stroke patients, 2011. www.clinicaltrials.gov (accessed 31 August 2012). [NCT01278498]
-
- Mead GE, Dennis MS, Innes K, MacLeod M, Sandercock PAG, House A, et al. A multicentre randomised trial to establish the effect(s) of routine administration of fluoxetine in patients with a recent stroke (Fluoxetine Or Control Under Supervision, FOCUS). Proceedings of the 21st European Stroke Conference 2012 (Abst. OAID 26). 2012.
Additional references
-
- Anonymous. Escitalopram improves global cognitive functioning in stroke patients. Australian Journal of Pharmacy 2010;91(1083):86.
-
- Anonymous. Fluoxetine enhances motor recovery if given soon after an ischaemic stroke. Australian Journal of Pharmacy 2011;92:91.
-
- Berends HI, Nijlant JMM, Movig KLLL, Putten MJAM, Jannink MJA, IJzerman MJ. The clinical use of drugs in influencing neurotransmitters in the brain to promote motor recovery after stroke: a Cochrane systematic review. European Journal of Physical and Rehabilitation Medicine 2009;45:621‐30. - PubMed
-
- Berends HI, IJzerman MI, Movig KLL, Putten MJAM. Long term administration of fluoxetine to improve motor recovery after stroke. Future Neurology 2011;6(4):455‐7.
-
- Bhogal SK, Teasell R, Foley N, Speechley M. Heterocyclics and selective serotonin reuptake inhibitors in the treatment and prevention of post‐stroke depression. Journal of the American Geriatrics Society 2005;53:1051‐7. - PubMed

