Epstein-Barr virus LMP1 modulates lipid raft microdomains and the vimentin cytoskeleton for signal transduction and transformation
- PMID: 23152522
- PMCID: PMC3554135
- DOI: 10.1128/JVI.02519-12
Epstein-Barr virus LMP1 modulates lipid raft microdomains and the vimentin cytoskeleton for signal transduction and transformation
Abstract
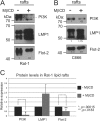
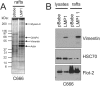
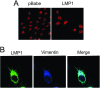
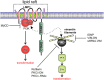
The Epstein-Barr virus (EBV) is an important human pathogen that is associated with multiple cancers. The major oncoprotein of the virus, latent membrane protein 1 (LMP1), is essential for EBV B-cell immortalization and is sufficient to transform rodent fibroblasts. This viral transmembrane protein activates multiple cellular signaling pathways by engaging critical effector molecules and thus acts as a ligand-independent growth factor receptor. LMP1 is thought to signal from internal lipid raft containing membranes; however, the mechanisms through which these events occur remain largely unknown. Lipid rafts are microdomains within membranes that are rich in cholesterol and sphingolipids. Lipid rafts act as organization centers for biological processes, including signal transduction, protein trafficking, and pathogen entry and egress. In this study, the recruitment of key signaling components to lipid raft microdomains by LMP1 was analyzed. LMP1 increased the localization of phosphatidylinositol 3-kinase (PI3K) and its activated downstream target, Akt, to lipid rafts. In addition, mass spectrometry analyses identified elevated vimentin in rafts isolated from LMP1 expressing NPC cells. Disruption of lipid rafts through cholesterol depletion inhibited PI3K localization to membranes and decreased both Akt and ERK activation. Reduction of vimentin levels or disruption of its organization also decreased LMP1-mediated Akt and ERK activation and inhibited transformation of rodent fibroblasts. These findings indicate that LMP1 reorganizes membrane and cytoskeleton microdomains to modulate signal transduction.
Figures





References
-
- Raab-Traub N. 2007. EBV-induced oncogenesis, p 986–1006 In Arvin A, Campadelli-Fiume G, Mocarski E, Moore PS, Roizman B, Whitley R, Yamanishi K. (ed), Human herpesviruses: biology, therapy, and immunoprophylaxis. Cambridge University Press, Cambridge, United Kingdom - PubMed
-
- Wang D, Liebowitz D, Kieff E. 1985. An EBV membrane protein expressed in immortalized lymphocytes transforms established rodent cells. Cell 43:831–840 - PubMed
-
- Mei Y-P, Zhou J-M, Wang Y, Huang H, Deng R, Feng G-K, Zeng Y-X, Zhu X-F. 2007. Silencing of LMP1 induces cell cycle arrest and enhances chemosensitivity through inhibition of AKT signaling pathway in EBV-positive nasopharyngeal carcinoma cells. Cell Cycle 6:1379–1385 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

