Novel, potentially zoonotic paramyxoviruses from the African straw-colored fruit bat Eidolon helvum
- PMID: 23152534
- PMCID: PMC3554137
- DOI: 10.1128/JVI.01202-12
Novel, potentially zoonotic paramyxoviruses from the African straw-colored fruit bat Eidolon helvum
Abstract
Bats carry a variety of paramyxoviruses that impact human and domestic animal health when spillover occurs. Recent studies have shown a great diversity of paramyxoviruses in an urban-roosting population of straw-colored fruit bats in Ghana. Here, we investigate this further through virus isolation and describe two novel rubulaviruses: Achimota virus 1 (AchPV1) and Achimota virus 2 (AchPV2). The viruses form a phylogenetic cluster with each other and other bat-derived rubulaviruses, such as Tuhoko viruses, Menangle virus, and Tioman virus. We developed AchPV1- and AchPV2-specific serological assays and found evidence of infection with both viruses in Eidolon helvum across sub-Saharan Africa and on islands in the Gulf of Guinea. Longitudinal sampling of E. helvum indicates virus persistence within fruit bat populations and suggests spread of AchPVs via horizontal transmission. We also detected possible serological evidence of human infection with AchPV2 in Ghana and Tanzania. It is likely that clinically significant zoonotic spillover of chiropteran paramyxoviruses could be missed throughout much of Africa where health surveillance and diagnostics are poor and comorbidities, such as infection with HIV or Plasmodium sp., are common.
Figures
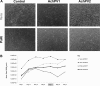

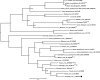


Comment in
-
Bat trait, genetic and pathogen data from large-scale investigations of African fruit bats, Eidolon helvum.Sci Data. 2016 Aug 1;3:160049. doi: 10.1038/sdata.2016.49. Sci Data. 2016. PMID: 27479120 Free PMC article.
References
-
- Drexler JF, Corman VM, Muller MA, Maganga GD, Vallo P, Binger T, Gloza-Rausch F, Rasche A, Yordanov S, Seebens A, Oppong S, Sarkodie YA, Pongombo C, Lukashev AN, Schmidt-Chanasit J, Stocker A, Carneiro AJ, Erbar S, Maisner A, Fronhoffs F, Buettner R, Kalko EK, Kruppa T, Franke CR, Kallies R, Yandoko ER, Herrler G, Reusken C, Hassanin A, Kruger DH, Matthee S, Ulrich RG, Leroy EM, Drosten C. 2012. Bats host major mammalian paramyxoviruses. Nat. Commun. 3:796. - PMC - PubMed
-
- Drexler JF, Corman VM, Gloza-Rausch F, Seebens A, Annan A, Ipsen A, Kruppa T, Muller MA, Kalko EK, Adu-Sarkodie Y, Oppong S, Drosten C. 2009. Henipavirus RNA in African bats. PLoS One 4:e6367 doi:10.1371/journal.pone.0006367 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

