Novel mutation in VCP gene causes atypical amyotrophic lateral sclerosis
- PMID: 23152587
- PMCID: PMC3570818
- DOI: 10.1212/WNL.0b013e318275963b
Novel mutation in VCP gene causes atypical amyotrophic lateral sclerosis
Abstract
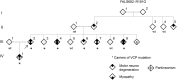
Objective: To identify the genetic variant that causes autosomal dominantly inherited motor neuron disease in a 4-generation Israeli-Arab family using genetic linkage and whole exome sequencing.
Methods: Genetic linkage analysis was performed in this family using Illumina single nucleotide polymorphism chips. Whole exome sequencing was then undertaken on DNA samples from 2 affected family members using an Illumina 2000 HiSeq platform in pursuit of potentially pathogenic genetic variants that comigrate with the disease in this pedigree. Variants meeting these criteria were then screened in all affected individuals.
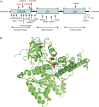
Results: A novel mutation (p.R191G) in the valosin-containing protein (VCP) gene was identified in the index family. Direct sequencing of the VCP gene in a panel of DNA from 274 unrelated individuals with familial amyotrophic lateral sclerosis (FALS) revealed 5 additional mutations. Among them, 2 were previously identified in pedigrees with a constellation of inclusion body myopathy with Paget disease of the bone and frontotemporal dementia (IBMPFD) and in FALS, and 2 other mutations (p.R159C and p.R155C) in IBMPFD alone. We did not detect VCP gene mutations in DNA from 178 cases of sporadic amyotrophic lateral sclerosis.
Conclusions: We report a novel VCP mutation identified in an amyotrophic lateral sclerosis family (p.R191G) with atypical clinical features. In our experience, VCP mutations arise in approximately 1.5% of FALS cases. Our study supports the view that motor neuron disease is part of the clinical spectrum of VCP-associated disease.
Figures
References
-
- Watts GD, Wymer J, Kovach MJ, et al. Inclusion body myopathy associated with Paget disease of bone and frontotemporal dementia is caused by mutant valosin-containing protein. Nat Genet 2004;36:377–381 - PubMed
-
- Haubenberger D, Bittner RE, Rauch-Schorny S, et al. Inclusion body myopathy and Paget disease is linked to a novel mutation in the VCP gene. Neurology 2005;65:1304–1305 - PubMed
-
- Guyant-Marèchal L, Laquerrière A, Duyckaerts C, et al. Valosin-containing protein gene mutations: clinical and neuropathological features. Neurology 2006;67:644–651 - PubMed
-
- Watts GD, Thomasova D, Ramdean SK, et al. Novel VCP mutations in inclusion body myopathy associated with Paget disease of bone and frontotemporal dementia. Clin Genet 2007;72:420–426 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
