Morphine withdrawal enhances constitutive μ-opioid receptor activity in the ventral tegmental area
- PMID: 23152596
- PMCID: PMC6794017
- DOI: 10.1523/JNEUROSCI.1572-12.2012
Morphine withdrawal enhances constitutive μ-opioid receptor activity in the ventral tegmental area
Abstract
μ-Opioid receptors (MORs) in the ventral tegmental area (VTA) are pivotally involved in addictive behavior. While MORs are typically activated by opioids, they can also become constitutively active in the absence of any agonist. In the current study, we present evidence that MOR constitutive activity is highly relevant in the mouse VTA, as it regulates GABAergic input to dopamine neurons. Specifically, suppression of MOR constitutive activity with the inverse agonist KC-2-009 enhanced GABAergic neurotransmission onto VTA dopamine neurons. This inverse agonistic effect was fully blocked by the specific MOR neutral antagonist CTOP, which had no effect on GABAergic transmission itself. We next show that withdrawal from chronic morphine further increases the magnitude of inverse agonistic effects at the MOR, suggesting enhanced MOR constitutive activity. We demonstrate that this increase can be an adaptive response to the detrimental elevation in cAMP levels known to occur during morphine withdrawal. These findings offer important insights in the physiological occurrence and function of MOR constitutive activity, and have important implications for therapeutic strategies aimed at normalizing MOR signaling during addiction and opioid overdose.
Figures

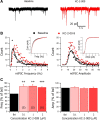



References
-
- Acquas E, Carboni E, Di Chiara G. Profound depression of mesolimbic dopamine release after morphine withdrawal in dependent rats. Eur J Pharmacol. 1991;193:133–134. - PubMed
-
- Aston-Jones G, Harris GC. Brain substrates for increased drug seeking during protracted withdrawal. Neuropharmacology. 2004;47(Suppl 1):167–179. - PubMed
-
- Bagley EE, Hacker J, Chefer VI, Mallet C, McNally GP, Chieng BC, Perroud J, Shippenberg TS, Christie MJ. Drug-induced GABA transporter currents enhance GABA release to induce opioid withdrawal behaviors. Nat Neurosci. 2011;14:1548–1554. - PubMed
-
- Baumeister AA, Anticich TG, Hebert G, Hawkins MF, Nagy M. Evidence that physical dependence on morphine is mediated by the ventral midbrain. Neuropharmacology. 1989;28:1151–1157. - PubMed
-
- Bergevin A, Girardot D, Bourque MJ, Trudeau LE. Presynaptic mu-opioid receptors regulate a late step of the secretory process in rat ventral tegmental area GABAergic neurons. Neuropharmacology. 2002;42:1065–1078. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
