Targeting astrocytes ameliorates neurologic changes in a mouse model of Alzheimer's disease
- PMID: 23152597
- PMCID: PMC3506017
- DOI: 10.1523/JNEUROSCI.2323-12.2012
Targeting astrocytes ameliorates neurologic changes in a mouse model of Alzheimer's disease
Abstract
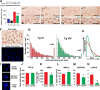
Astrocytes are the most abundant cell type in the brain and play a critical role in maintaining healthy nervous tissue. In Alzheimer's disease (AD) and most other neurodegenerative disorders, many astrocytes convert to a chronically "activated" phenotype characterized by morphologic and biochemical changes that appear to compromise protective properties and/or promote harmful neuroinflammatory processes. Activated astrocytes emerge early in the course of AD and become increasingly prominent as clinical and pathological symptoms progress, but few studies have tested the potential of astrocyte-targeted therapeutics in an intact animal model of AD. Here, we used adeno-associated virus (AAV) vectors containing the astrocyte-specific Gfa2 promoter to target hippocampal astrocytes in APP/PS1 mice. AAV-Gfa2 vectors drove the expression of VIVIT, a peptide that interferes with the immune/inflammatory calcineurin/NFAT (nuclear factor of activated T-cells) signaling pathway, shown by our laboratory and others to orchestrate biochemical cascades leading to astrocyte activation. After several months of treatment with Gfa2-VIVIT, APP/PS1 mice exhibited improved cognitive and synaptic function, reduced glial activation, and lower amyloid levels. The results confirm a deleterious role for activated astrocytes in AD and lay the groundwork for exploration of other novel astrocyte-based therapies.
Figures






References
-
- Agostinho P, Lopes JP, Velez Z, Oliveira CR. Overactivation of calcineurin induced by amyloid-beta and prion proteins. Neurochem Int. 2008;52:1226–1233. - PubMed
-
- Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, Finch CE, Frautschy S, Griffin WS, Hampel H, Hull M, Landreth G, Lue L, Mrak R, Mackenzie IR, McGeer PL, O'Banion MK, et al. Inflammation and Alzheimer's disease. Neurobiol Aging. 2000;21:383–421. - PMC - PubMed
-
- Aramburu J, Yaffe MB, López-Rodríguez C, Cantley LC, Hogan PG, Rao A. Affinity-driven peptide selection of an NFAT inhibitor more selective than cyclosporin A. Science. 1999;285:2129–2133. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
