β-secretase cleavage of the fly amyloid precursor protein is required for glial survival
- PMID: 23152602
- PMCID: PMC3936607
- DOI: 10.1523/JNEUROSCI.0228-12.2012
β-secretase cleavage of the fly amyloid precursor protein is required for glial survival
Abstract
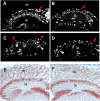
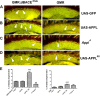
β-secretase (or BACE1) is the key enzyme in the production of β-amyloid (Aβ), which accumulates in the senile plaques characteristic for Alzheimer's disease. Consequently, the lack of BACE1 prevents β-processing of the amyloid precursor protein and Aβ production, which made it a promising target for drug development. However, the loss of BACE1 is also detrimental, leading to myelination defects and altered neuronal activity, functions that have been associated with the cleavage of Neuregulin and a voltage-gated sodium channel subunit. Here we show that the Drosophila ortholog of BACE, dBACE, is required for glial survival. Cell-specific knockdown experiments reveal that this is a non-cell autonomous function, as a knockdown of dBACE in photoreceptor neurons leads to progressive degeneration of glia in their target zone, the lamina. Interestingly, this phenotype is suppressed by the loss of the fly amyloid precursor protein (APPL), whereas a secretion-deficient form of APPL enhances the degeneration. This shows that full-length APPL in neurons promotes the death of neighboring glial cells and that β-processing of APPL is needed to prevent glial death. These results therefore not only demonstrate a novel function for an APP protein in glia, but they also show this function specifically requires regulation by β-cleavage.
Figures
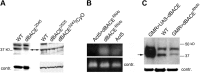
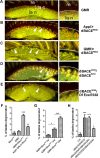
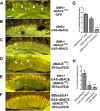
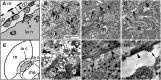


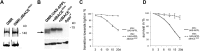
References
-
- Bennett BD, Babu-Khan S, Loeloff R, Louis JC, Curran E, Citron M, Vassar R. Expression analysis of BACE2 in brain and peripheral tissues. J Biol Chem. 2000;275:20647–20651. - PubMed
-
- Bettencourt da Cruz A, Schwärzel M, Schulze S, Niyyati M, Heisenberg M, Kretzschmar D. Disruption of the MAP1B-related protein FUTSCH leads to changes in the neuronal cytoskeleton, axonal transport defects, and progressive neurodegeneration in Drosophila. Mol Biol Cell. 2005;16:2433–2442. - PMC - PubMed
-
- Bogart K, Andrews J. CGB Technical Report 10. Bloomington, IN: The Center for Genomics and Bioinformatics; 2006. Extraction of Total RNA from Drosophila.
-
- Cai H, Wang Y, McCarthy D, Wen H, Borchelt DR, Price DL, Wong PC. BACE1 is the major beta-secretase for generation of Abeta peptides by neurons. Nat Neurosci. 2001;4:233–234. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
