Aging differentially affects the re-entrainment response of central and peripheral circadian oscillators
- PMID: 23152603
- PMCID: PMC3507430
- DOI: 10.1523/JNEUROSCI.3559-12.2012
Aging differentially affects the re-entrainment response of central and peripheral circadian oscillators
Abstract
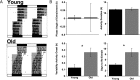
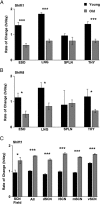
Aging produces a decline in the amplitude and precision of 24 h behavioral, endocrine, and metabolic rhythms, which are regulated in mammals by a central circadian pacemaker within the suprachiasmatic nucleus (SCN) and local oscillators in peripheral tissues. Disruption of the circadian system, as experienced during transmeridian travel, can lead to adverse health consequences, particularly in the elderly. To test the hypothesis that age-related changes in the response to simulated jet lag will reflect altered circadian function, we examined re-entrainment of central and peripheral oscillators from young and old PER2::luciferase mice. As in previous studies, locomotor activity rhythms in older mice required more days to re-entrain following a shift than younger mice. At the tissue level, effects of age on baseline entrainment were evident, with older mice displaying earlier phases for the majority of peripheral oscillators studied and later phases for cells within most SCN subregions. Following a 6 h advance of the light:dark cycle, old mice displayed slower rates of re-entrainment for peripheral tissues but a larger, more rapid SCN response compared to younger mice. Thus, aging alters the circadian timing system in a manner that differentially affects the re-entrainment responses of central and peripheral circadian clocks. This pattern of results suggests that a major consequence of aging is a decrease in pacemaker amplitude, which would slow re-entrainment of peripheral oscillators and reduce SCN resistance to external perturbation.
Figures




References
-
- Ancoli-Israel S, Ayalon L. Diagnosis and treatment of sleep disorders in older adults. Am J Geriatr Psychiatry. 2006;14:95–103. - PubMed
-
- Antle MC, Silver R. Orchestrating time: arrangements of the brain circadian clock. Trends Neurosci. 2005;28:145–151. - PubMed
-
- Arendt J. Managing jet lag: some of the problems and possible new solutions. Sleep Med Rev. 2009;13:249–256. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
