Enhancing microparticle internalization by nonphagocytic cells through the use of noncovalently conjugated polyethyleneimine
- PMID: 23152683
- PMCID: PMC3496409
- DOI: 10.2147/IJN.S34635
Enhancing microparticle internalization by nonphagocytic cells through the use of noncovalently conjugated polyethyleneimine
Abstract
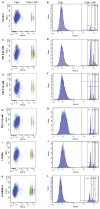
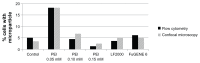
Development of micro- and nanotechnology for the study of living cells, especially in the field of drug delivery, has gained interest in recent years. Although several studies have reported successful results in the internalization of micro- and nanoparticles in phagocytic cells, when nonphagocytic cells are used, the low internalization efficiency represents a limitation that needs to be overcome. It has been reported that covalent surface modification of micro- and nanoparticles increases their internalization rate. However, this surface modification represents an obstacle for their use as drug-delivery carriers. For this reason, the aim of the present study was to increase the capability for microparticle internalization of HeLa cells through the use of noncovalently bound transfection reagents: polyethyleneimine (PEI) Lipofectamine™ 2000 and FuGENE 6(®). Both confocal microscopy and flow cytometry techniques allowed us to precisely quantify the efficiency of microparticle internalization by HeLa cells, yielding similar results. In addition, intracellular location of microparticles was analyzed through transmission electron microscopy and confocal microscopy procedures. Our results showed that free PEI at a concentration of 0.05 mM significantly increased microparticle uptake by cells, with a low cytotoxic effect. As determined by transmission electron and confocal microscopy analyses, microparticles were engulfed by plasma-membrane projections during internalization, and 24 hours later they were trapped in a lysosomal compartment. These results show the potential use of noncovalently conjugated PEI in microparticle internalization assays.
Keywords: HeLa cells; drug delivery; endocytosis; internalization efficiency.
Figures







References
-
- Grayson ACR, Shawgo RS, Johnson AM, et al. A BioMEMS review: MEMS technology for physiologically integrated devices. Proc IEEE. 2004;92(1):6–21.
-
- Fernandez-Rosas E, Gómez R, Ibanez E, et al. Intracellular polysilicon barcodes for cell tracking. Small. 2009;5(21):2433–2439. - PubMed
-
- Novo S, Barrios L, Santaló J, et al. A novel embryo identification system by direct tagging of mouse embryos using silicon-based barcodes. Hum Reprod. 2011;26(1):96–105. - PubMed
-
- Tao Z, Toms B, Goodisman J, Asefa T. Mesoporous silica microparticles enhance the cytotoxicity of anticancer platinum drugs. ACS Nano. 2010;4(2):789–794. - PubMed
-
- Tasciotti E, Liu X, Bhavane R, et al. Mesoporous silicon particles as a multistage delivery system for imaging and therapeutic applications. Nat Nanotechnol. 2008;3(3):151–157. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

