Juvéderm(®) Volbella™ in the perioral area: a 12-month prospective, multicenter, open-label study
- PMID: 23152693
- PMCID: PMC3496328
- DOI: 10.2147/CCID.S35800
Juvéderm(®) Volbella™ in the perioral area: a 12-month prospective, multicenter, open-label study
Abstract
Background: Hyaluronic acid dermal fillers are frequently used for lip augmentation, and a new filler has been developed with characteristics especially suited for the lips.
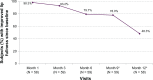
Methods: Four European sites treated 60 subjects with Juvéderm(®) Volbella™ injectable gel in the perioral area, and subjects returned to the clinic at 1, 3, 6, 9, and 12 months for follow-up. The primary effectiveness endpoint established a priori was a Month 3 responder rate on the 4-point Lip Fullness Scale (LFS) of ≥40% and statistically > 0%, where responders improved ≥ 1 point from baseline on the investigator's assessment of LFS. At follow-up, subjects assessed lip fullness goal achievement, the look and feel of their lips, and their satisfaction with the effects of treatment.
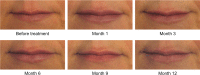
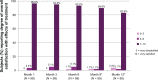
Results: The Month 3 LFS responder rate was 93.2% (P < 0.0001), so the primary endpoint was met, and clinical effectiveness was demonstrated. The responder rate over time showed that 78.0% of subjects still had improved lip fullness at Month 9 and 48.3% at Month 12. After treatment 98.3% of subjects reported that their lip fullness goal had been achieved, and this was maintained at 86.4% at Month 9 and 56.9% at Month 12. At Month 1, 81.0% of subjects reported that their lips felt smooth, and 91.4% reported that their lips looked natural (scores of 7-10 on an 11-point scale, where 0 was an unfavorable outcome and 10 was a favorable outcome). Similarly, 96.6% of subjects reported being satisfied (scores between 7 and 10 on an 11-point scale where 0 = very dissatisfied, 10 = very satisfied) at Month 1, and by Month 12 more than 80% of subjects were still satisfied. There were no severe adverse events related to treatment.
Conclusion: Juvéderm(®) Volbella™ injectable gel is well tolerated and has been demonstrated to provide a smooth and natural improvement in lip fullness that lasts for up to 1 year.
Keywords: dermal filler; hyaluronic acid; lips; patient satisfaction.
Figures



Similar articles
-
A prospective, open-label, multicenter, observational, postmarket study of the use of a 15 mg/mL hyaluronic acid dermal filler in the lips.J Cosmet Dermatol. 2014 Jun;13(2):125-34. doi: 10.1111/jocd.12085. J Cosmet Dermatol. 2014. PMID: 24910276 Free PMC article.
-
Safety and Effectiveness of the Hyaluronic Acid Filler, HYC-24L, for Lip and Perioral Augmentation.Dermatol Surg. 2015 Dec;41 Suppl 1:S293-301. doi: 10.1097/DSS.0000000000000540. Dermatol Surg. 2015. PMID: 26618456 Clinical Trial.
-
Safety and Effectiveness of Repeat Treatment With VYC-15L for Lip and Perioral Enhancement: Results From a Prospective Multicenter Study.Aesthet Surg J. 2019 Mar 14;39(4):413-422. doi: 10.1093/asj/sjy019. Aesthet Surg J. 2019. PMID: 29506034 Free PMC article. Clinical Trial.
-
Hyaluronic Acid Is an Effective Dermal Filler for Lip Augmentation: A Meta-Analysis.Front Surg. 2021 Aug 6;8:681028. doi: 10.3389/fsurg.2021.681028. eCollection 2021. Front Surg. 2021. PMID: 34422892 Free PMC article.
-
Effectiveness and safety of hyaluronic acid fillers used to enhance overall lip fullness: A systematic review of clinical studies.J Cosmet Dermatol. 2019 Apr;18(2):436-443. doi: 10.1111/jocd.12861. Epub 2019 Jan 12. J Cosmet Dermatol. 2019. PMID: 30636365
Cited by
-
Discussion: juvéderm volbella with lidocaine for lip and perioral enhancement: a prospective, randomized, controlled trial.Plast Reconstr Surg Glob Open. 2015 Apr 7;3(3):e322. doi: 10.1097/GOX.0000000000000274. eCollection 2015 Mar. Plast Reconstr Surg Glob Open. 2015. PMID: 25878933 Free PMC article. No abstract available.
-
A Prospective, Open-Label, Multicenter, Real-World Study of VYC-17.5L Hyaluronic Acid Dermal Filler in the Lips.Aesthet Surg J Open Forum. 2022 May 15;4:ojac047. doi: 10.1093/asjof/ojac047. eCollection 2022. Aesthet Surg J Open Forum. 2022. PMID: 35795884 Free PMC article.
-
Juvéderm volbella with lidocaine for lip and perioral enhancement: a prospective, randomized, controlled trial.Plast Reconstr Surg Glob Open. 2015 Apr 7;3(3):e321. doi: 10.1097/GOX.0000000000000266. eCollection 2015 Mar. Plast Reconstr Surg Glob Open. 2015. PMID: 25878932 Free PMC article.
-
Adverse effects of the aesthetic use of botulinum toxin and dermal fillers on the face: a narrative review.An Bras Dermatol. 2025 Jan-Feb;100(1):87-103. doi: 10.1016/j.abd.2024.04.007. Epub 2024 Nov 29. An Bras Dermatol. 2025. PMID: 39616095 Free PMC article. Review.
-
Assessment of the Efficacy and Safety of Hyaluronic Acid Gel Injection in the Restoration of Fullness of the Upper Lips.J Cutan Aesthet Surg. 2017 Apr-Jun;10(2):101-105. doi: 10.4103/JCAS.JCAS_115_16. J Cutan Aesthet Surg. 2017. PMID: 28852297 Free PMC article.
References
-
- Borrell M, Leslie DB, Tezel A. Lift capabilities of hyaluronic acid fillers. J Cosmet Laser Ther. 2011;13(1):21–27. - PubMed
-
- Fagien S, Maas C, Murphy DK, Thomas JA, Beddingfield FC, for the Juvéderm Lips Study Group Juvéderm® Ultra for lip enhancement: an open-label, multi-center study. Aesththetic Surgery Journal. 2012 In press; - PubMed
-
- Solish N, Swift A. An open-label, pilot study to assess the effectiveness and safety of hyaluronic acid gel in the restoration of soft tissue fullness of the lips. J Drugs Dermatol. 2011;10(2):145–149. - PubMed
-
- Restylane® instructions for use [package insert] Scottsdale, AZ: Medicis Aesthetics Inc; 2011.
-
- Glogau RG, Bank D, Brandt F, et al. A randomized, evaluator-blinded, controlled study of the effectiveness and safety of small gel particle hyaluronic acid for lip augmentation. Dermatol Surg. 2012;38(7 Pt 2):1180–1192. - PubMed
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

