Knockdown of Ki-67 by dicer-substrate small interfering RNA sensitizes bladder cancer cells to curcumin-induced tumor inhibition
- PMID: 23152782
- PMCID: PMC3495973
- DOI: 10.1371/journal.pone.0048567
Knockdown of Ki-67 by dicer-substrate small interfering RNA sensitizes bladder cancer cells to curcumin-induced tumor inhibition
Abstract
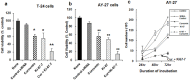
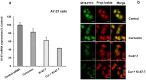
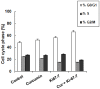
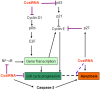
Transitional cell carcinoma (TCC) of the urinary bladder is the most common cancer of the urinary tract. Most of the TCC cases are of the superficial type and are treated with transurethral resection (TUR). However, the recurrence rate is high and the current treatments have the drawback of inducing strong systemic toxicity or cause painful cystitis. Therefore, it would be of therapeutic value to develop novel concepts and identify novel drugs for the treatment of bladder cancer. Ki-67 is a large nucleolar phosphoprotein whose expression is tightly linked to cell proliferation, and curcumin, a phytochemical derived from the rhizome Curcuma longa, has been shown to possess powerful anticancer properties. In this study, we evaluated the combined efficacy of curcumin and a siRNA against Ki-67 mRNA (Ki-67-7) in rat (AY-27) and human (T-24) bladder cancer cells. The anticancer effects were assessed by the determination of cell viability, apoptosis and cell cycle analysis. Ki-67-7 (10 nM) and curcumin (10 µM), when treated independently, were moderately effective. However, in their combined presence, proliferation of bladder cancer cells was profoundly (>85%) inhibited; the rate of apoptosis in the combined presence of curcumin and Ki-67-7 (36%) was greater than that due to Ki-67-7 (14%) or curcumin (13%) alone. A similar synergy between curcumin and Ki-67-7 in inducing cell cycle arrest was also observed. Western blot analysis suggested that pretreatment with Ki-67-7 sensitized bladder cancer cells to curcumin-mediated apoptosis and cell cycle arrest by p53- and p21-independent mechanisms. These data suggest that a combination of anti-Ki-67 siRNA and curcumin could be a viable treatment against the proliferation of bladder cancer cells.
Conflict of interest statement
Figures




References
-
- Parker SL, Tong T, Bolden S, Wingo PA (1996) Cancer Statistics. CA Cancer J Clin: 5–27. - PubMed
-
- Lamm DL, Riggs DR, Traynelis CL, Nseyo UO (1995) Apparent failure of current intravesical chemotherapy prophylaxis to influence long-term course of superficial transitional cell carcinoma of the bladder. J Urol 153: 1444–1450. - PubMed
-
- Parkin DM, Bray F, Ferlay J, Pisani P (2005) Global Cancer Statistics, 2002. CA Cancer J Clin 55: 74–108. - PubMed
-
- Herr HW, Donat SM, Reuter VE (2007) Management of low grade papillary bladder tumors. J Urol 178: 1201–1205. - PubMed
-
- Guhasarkar S, Banerjee R (2010) Intravesical drug delivery: Challenges, current status, opportunities and novel strategies. J Control Release 48: 147–159. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

