Targeting the acute promyelocytic leukemia-associated fusion proteins PML/RARα and PLZF/RARα with interfering peptides
- PMID: 23152790
- PMCID: PMC3494703
- DOI: 10.1371/journal.pone.0048636
Targeting the acute promyelocytic leukemia-associated fusion proteins PML/RARα and PLZF/RARα with interfering peptides
Abstract
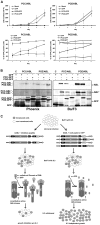
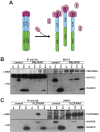
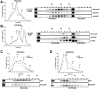
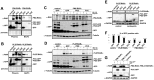
In acute promyelocytic leukemia (APL), hematopoietic differentiation is blocked and immature blasts accumulate in the bone marrow and blood. APL is associated with chromosomal aberrations, including t(15;17) and t(11;17). For these two translocations, the retinoic acid receptor alpha (RARα) is fused to the promyelocytic leukemia (PML) gene or the promyelocytic zinc finger (PLZF) gene, respectively. Both fusion proteins lead to the formation of a high-molecular-weight complex. High-molecular-weight complexes are caused by the "coiled-coil" domain of PML or the BTB/POZ domain of PLZF. PML/RARα without the "coiled-coil" fails to block differentiation and mediates an all-trans retinoic acid-response. Similarly, mutations in the BTB/POZ domain disrupt the high-molecular-weight complex, abolishing the leukemic potential of PLZF/RARα. Specific interfering polypeptides were used to target the oligomerization domain of PML/RARα or PLZF/RARα. PML/RARα and PLZF/RARα were analyzed for the ability to form high-molecular-weight complexes, the protein stability and the potential to induce a leukemic phenotype in the presence of the interfering peptides. Expression of these interfering peptides resulted in a reduced replating efficiency and overcame the differentiation block induced by PML/RARα and PLZF/RARα in murine hematopoietic stem cells. This expression also destabilized the PLZF/RARα-induced high-molecular-weight complex formation and caused the degradation of the fusion protein. Targeting fusion proteins through interfering peptides is a promising approach to further elucidate the biology of leukemia.
Conflict of interest statement
Figures


References
-
- Puccetti E, Ruthardt M (2004) Acute promyelocytic leukemia: PML/RARalpha and the leukemic stem cell. Leukemia 18: 1169–1175. - PubMed
-
- He LZ, Merghoub T, Pandolfi PP (1999) In vivo analysis of the molecular pathogenesis of acute promyelocytic leukemia in the mouse and its therapeutic implications. Oncogene 18: 5278–5292. - PubMed
-
- Puccetti E, Obradovic D, Beissert T, Bianchini A, Washburn B, et al. (2002) AML-associated translocation products block vitamin D(3)-induced differentiation by sequestering the vitamin D(3) receptor. Cancer Res 62: 7050–7058. - PubMed
-
- Seshire A, Rossiger T, Frech M, Beez S, Hagemeyer H, et al. (2012) Direct interaction of PU.1 with oncogenic transcription factors reduces its serine phosphorylation and promoter binding. Leukemia doi:10.1038/leu.2011.331. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

