Inhibition of cyclooxygenase-2 reduces hypothalamic excitation in rats with adriamycin-induced heart failure
- PMID: 23152801
- PMCID: PMC3496718
- DOI: 10.1371/journal.pone.0048771
Inhibition of cyclooxygenase-2 reduces hypothalamic excitation in rats with adriamycin-induced heart failure
Abstract
Background: The paraventricular nucleus (PVN) of the hypothalamus plays an important role in the progression of heart failure (HF). We investigated whether cyclooxygenase-2 (COX-2) inhibition in the PVN attenuates the activities of sympathetic nervous system (SNS) and renin-angiotensin system (RAS) in rats with adriamycin-induced heart failure.
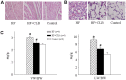
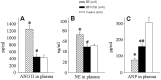
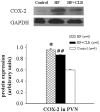
Methodology/principal finding: Heart failure was induced by intraperitoneal injection of adriamycin over a period of 2 weeks (cumulative dose of 15 mg/kg). On day 19, rats received intragastric administration daily with either COX-2 inhibitor celecoxib (CLB) or normal saline. Treatment with CLB reduced mortality and attenuated both myocardial atrophy and pulmonary congestion in HF rats. Compared with the HF rats, ventricle to body weight (VW/BW) and lung to body weight (LW/BW) ratios, heart rate (HR), left ventricular end-diastolic pressure (LVEDP), left ventricular peak systolic pressure (LVPSP) and maximum rate of change in left ventricular pressure (LV±dp/dtmax) were improved in HF+CLB rats. Angiotensin II (ANG II), norepinephrine (NE), COX-2 and glutamate (Glu) in the PVN were increased in HF rats. HF rats had higher levels of ANG II and NE in plasma, higher level of ANG II in myocardium, and lower levels of ANP in plasma and myocardium. Treatment with CLB attenuated these HF-induced changes. HF rats had more COX-2-positive neurons and more corticotropin releasing hormone (CRH) positive neurons in the PVN than did control rats. Treatment with CLB decreased COX-2-positive neurons and CRH positive neurons in the PVN of HF rats.
Conclusions: These results suggest that PVN COX-2 may be an intermediary step for PVN neuronal activation and excitatory neurotransmitter release, which further contributes to sympathoexcitation and RAS activation in adriamycin-induced heart failure. Treatment with COX-2 inhibitor attenuates sympathoexcitation and RAS activation in adriamycin-induced heart failure.
Conflict of interest statement
Figures




Similar articles
-
Inhibition of brain proinflammatory cytokine synthesis reduces hypothalamic excitation in rats with ischemia-induced heart failure.Am J Physiol Heart Circ Physiol. 2008 Jul;295(1):H227-36. doi: 10.1152/ajpheart.01157.2007. Epub 2008 May 16. Am J Physiol Heart Circ Physiol. 2008. PMID: 18487441 Free PMC article.
-
Paraventricular nucleus corticotrophin releasing hormone contributes to sympathoexcitation via interaction with neurotransmitters in heart failure.Basic Res Cardiol. 2011 May;106(3):473-83. doi: 10.1007/s00395-011-0155-2. Epub 2011 Feb 2. Basic Res Cardiol. 2011. PMID: 21287352 Free PMC article.
-
Effect of the changes of NMDA receptor in hypothalamic paraventricular nucleus on cardiac function and sympathetic nervous activity in rats with heart failure.Biochem Biophys Res Commun. 2017 Nov 25;493(3):1336-1341. doi: 10.1016/j.bbrc.2017.09.140. Epub 2017 Sep 27. Biochem Biophys Res Commun. 2017. PMID: 28958939
-
Hypothalamic paraventricular nucleus activation contributes to neurohumoral excitation in rats with heart failure.Regen Med Res. 2014 Jan 8;2(1):2. doi: 10.1186/2050-490X-2-2. eCollection 2014 Dec. Regen Med Res. 2014. PMID: 25984330 Free PMC article. Review.
-
Chronic heart failure: a disease of the brain.Heart Fail Rev. 2019 Mar;24(2):301-307. doi: 10.1007/s10741-018-9747-3. Heart Fail Rev. 2019. PMID: 30341700 Review.
Cited by
-
Lifestyle and Pharmacological Interventions to Prevent Anthracycline-Related Cardiotoxicity in Cancer Patients.J Cardiovasc Dev Dis. 2025 Jun 4;12(6):212. doi: 10.3390/jcdd12060212. J Cardiovasc Dev Dis. 2025. PMID: 40558647 Free PMC article. Review.
-
Does Myocardial Atrophy Represent Anti-Arrhythmic Phenotype?Biomedicines. 2022 Nov 4;10(11):2819. doi: 10.3390/biomedicines10112819. Biomedicines. 2022. PMID: 36359339 Free PMC article. Review.
-
Valproic Acid Prodrug Affects Selective Markers, Augments Doxorubicin Anticancer Activity and Attenuates Its Toxicity in a Murine Model of Aggressive Breast Cancer.Pharmaceuticals (Basel). 2021 Nov 30;14(12):1244. doi: 10.3390/ph14121244. Pharmaceuticals (Basel). 2021. PMID: 34959644 Free PMC article.
-
Astemizole Exacerbates 5-Fluorouracil-Triggered Cardiotoxicity by Enhancing Ptgs2.Cardiovasc Toxicol. 2025 Feb;25(2):205-215. doi: 10.1007/s12012-024-09953-3. Epub 2025 Jan 8. Cardiovasc Toxicol. 2025. PMID: 39779614
-
Cardiac fibrosis in oncologic therapies.Curr Opin Physiol. 2022 Oct;29:100575. doi: 10.1016/j.cophys.2022.100575. Epub 2022 Aug 8. Curr Opin Physiol. 2022. PMID: 36187050 Free PMC article.
References
-
- Francis J, Zhang ZH, Weiss RM, Felder RB (2004) Neural regulation of the proinflammatory cytokine response to acute myocardial infarction. Am J Physiol Heart Circ Physiol 287: H791–H797. - PubMed
-
- Zanzinger J, Czachurski J (2000) Chronic oxidative stress in the RVLM modulates sympathetic control of circulation in pigs. Pflugers Arch 439: 489–494. - PubMed
-
- Swanson LW, Sawchenko PE (1980) Paraventricular nucleus: a site for the integration of neuroendocrine and autonomic mechanisms. Neuroendocrinology 31: 410–417. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

